Fig. 2. Co-administration of the 11β-hydroxysteroid dehydrogenase type 1 inhibitor, AZD4017, limits the prednisolone-induced changes in insulin sensitivity (A and B) and glucose disposal (C and D) as measured across a 2-step hyperinsulinaemic euglycaemic clamp.
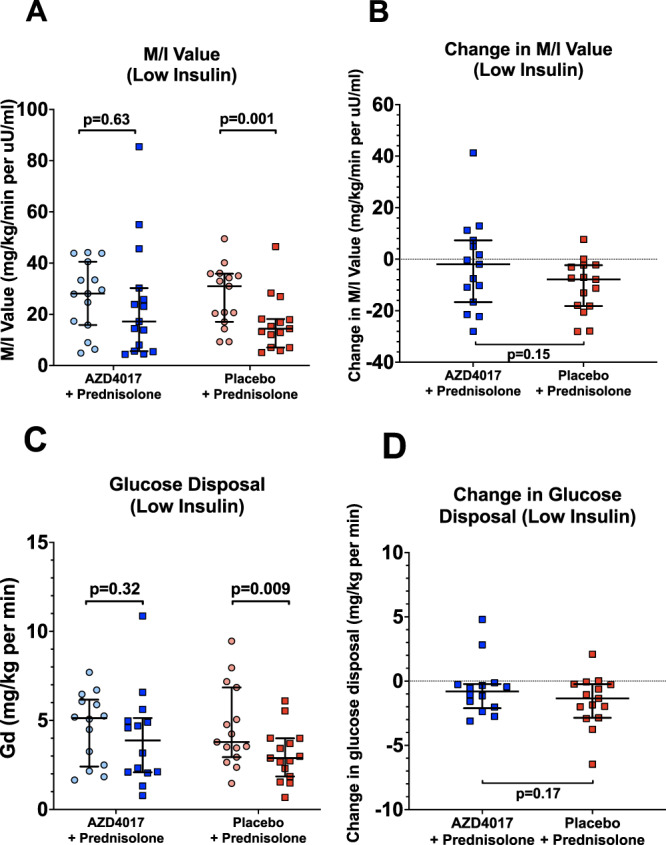
A Insulin sensitivity pre and post treatment in AZD4017+prednisolone and placebo+prednisolone groups. B Change in insulin sensitivity in AZD4017+prednisolone and placebo+prednisolone groups. C Glucose disposal pre and post treatment in AZD4017+prednisolone and placebo+prednisolone groups. D Change in glucose disposal in AZD4017+prednisolone and placebo+prednisolone groups. Data are medians and error bars are IQR. Data points represent individual patients. M/I value (Low insulin) AZD4017 + prednisolone n = 15, M/I value (Low insulin) placebo+prednisolone n = 15, Glucose disposal (Low insulin) AZD4017 + prednisolone n = 14, Glucose disposal (Low insulin) placebo+prednisolone n = 15. (Light blue circles = pre-treatment AZD4017 + prednisolone, blue squares = post-treatment AZD4017 + prednisolone, pink circles = pre-treatment placebo + prednisolone, red squares = post-treatment placebo + prednisolone). Statistical tests: Wilcoxon signed-rank test; Fig. 2A, generalised linear models adjusting for baseline variability in each specified outcome; Fig. 2B and D and paired two-tailed t-test; Fig. 2C.
