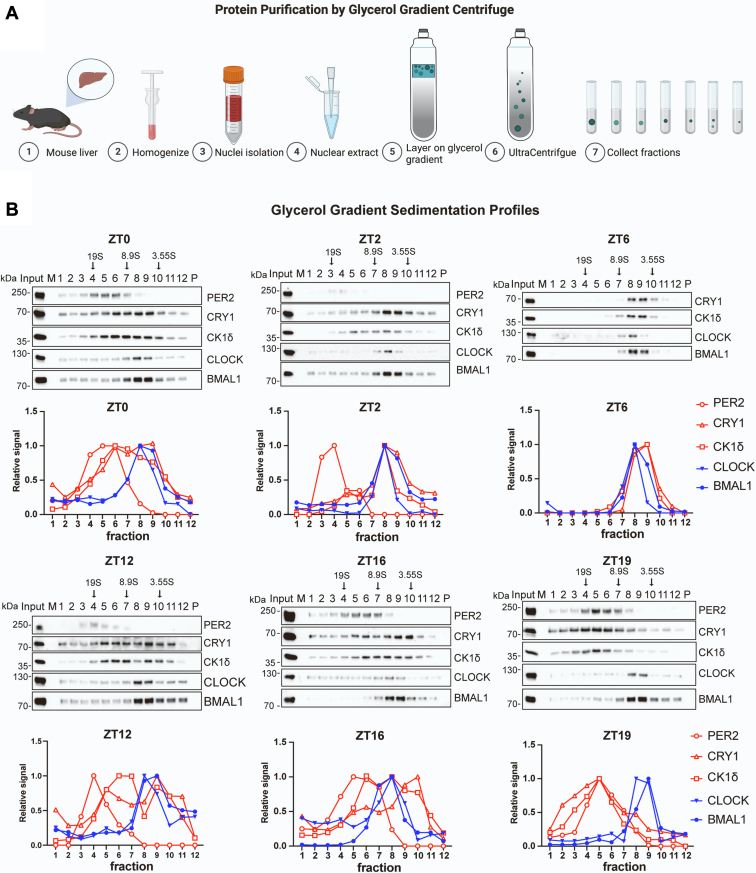
Figure 1.
Analysis of nuclear circadian complexes by glycerol gradient centrifugation.A, method: Mouse nuclear extract and reference proteins were mixed, layered on a 10 to 30% glycerol gradient, and centrifuged, and then fractions were collected from the bottom for analysis by SDS gel electrophoresis followed by Western blot (Fig. 1B) and Coomassie blue staining (Fig. S1). Reference proteins included bovine thyroglobulin (669 kDa, 19 S), sweet potato beta-amylase (222 kDa, 8.9 S), and chicken ovalbumin (43 kDa, 3.55 S). B, sedimentation profiles for PER2, CRY1, CK1δ, CLOCK, and BMAL1 determined for extracts of mice harvested at ZT0, ZT2, ZT6, ZT12, ZT16, and ZT19. For each ZT, the Western blot is above a graph showing quantitative values for band intensity of each protein relative to each protein’s peak intensity, given a value of 1. Arrows indicate positions of the peak fraction of each reference protein as determined from Coomassie blue–stained gels with the same samples (Fig. S1). In the blots, “P” stands for pellet; this sample was obtained by washing the emptied gradient tube with 240 ul buffer; the purpose of analyzing this sample was to detect and characterize any insoluble material that might pellet during centrifugation. No proteins were detected in the pellets. Three percent of each extract was loaded directly to the gels to indicate “Input.” Ten percent of each fraction was loaded to the gels. Three repeats were done for each ZT, and essentially identical data were obtained at each ZT. Representative images are shown.