Abstract
Introduction
Vaccination is a key strategy to limit the impact of the COVID-19 pandemic, among vulnerable groups such as cancer patients. However, COVID-19 vaccine hesitancy is limiting vaccination uptake in this population as in others. This study aimed to synthesise the emerging literature on vaccine hesitancy in this population and in Oncology health professionals, reasons for and factors associated with hesitancy, and interventions that address hesitancy.
Methods
A rapid review was undertaken PubMed, Ovid and Google across all years up to October 2021 for articles in English, from any country or region, addressing the above issues. Individual case studies, opinion pieces, commentary articles and conference abstracts were excluded. Article screening, data extraction and bias assessment were conducted by two authors. A narrative synthesis of the data was undertaken.
Results
Eighteen eligible articles were identified. Reported COVID-19 vaccine hesitancy rates varied from 76.7 % to 3.9 %, with a mean of 38.4 %. A large international study (n > 20,000) reported a more conservative hesitancy rate of 19 %. Six broad, common reasons for hesitancy were identified. Oncologist advice was valued by patients.
Discussion
Vaccine hesitancy remains a significant concern in the oncology context. Oncologists are key to addressing hesitancy and providing tailored advice to cancer patients.
Practice implications
Where possible, patients appreciate personalised, tailored information about vaccination which addresses its interaction with cancer and its treatment. Education programmes for oncologists to support effective communication in this context are needed. Webinars and peer-to-peer counselling may be useful but remain to be proven.
Keywords: COVID-19 vaccine hesitancy, Cancer patients
1. Introduction
COVID-19 has posed an immense challenge to health systems worldwide, causing high mortality and morbidity in the general population and workplace stress and burnout in healthcare workers [1]. Some populations are particularly vulnerable to COVID-19, including cancer patients whose treatment may leave them immunologically compromised. Individuals with cancer have been shown to have worse clinical outcomes and increased mortality from COVID-19, particularly those receiving active treatment and those with advanced cancer [2], [3], [4], [5].
The rapid development of vaccines has provided an effective strategy to combat the lethality and severity of COVID-19, reducing hospitalisations and relieving pressure on health workers [6]. This has led to a strong need for information about COVID-19 vaccines specifically in the context of cancer, from both patients and Oncology health professionals. However, it is not yet clear how COVID-19 vaccines may interact with cancer treatment schedules, side-effects and outcomes.
Vaccine hesitancy has led to a proportion of the population eschewing vaccination, including those with cancer [7]. Multiple factors have been shown to contribute to COVID-19 vaccine hesitancy, including concerns about the safety and efficacy of the vaccines, the process in which the vaccines were developed, and potential interactions between vaccine side-effects and other morbidities [8], [9].
It is unclear how cancer patients and Oncology health professionals are currently communicating and deciding about COVID-19 vaccination. While there is an emerging literature on vaccine hesitancy in the cancer context, a synthesis of findings is not yet available.
Thus, the aim of this study was to collate studies on vaccine hesitancy in the cancer context and synthesise the results. Following consultation with stakeholders (Oncology staff and consumers) we framed the following four research questions:
-
1.
Are cancer patients getting vaccinated at the same rate as the general population?
-
2.
Is there vaccine hesitancy among health professionals?
-
3.
Is there vaccine hesitancy among patients?
-
4.
Is there any evidence regarding strategies to address this vaccine hesitancy?
2. Methods
Due to the immediacy of need for guidance regarding COVID-19 vaccination in the cancer context, we chose to undertake a rapid review of the literature, “a form of knowledge synthesis that accelerates the process of conducting a traditional systematic review through streamlining or omitting specific methods to produce evidence for stakeholders in a resource-efficient manner.” [10]. We followed the Cochrane Rapid review Interim Recommendations [11] (see Supplementary Table 1).
2.1. Search strategy
A literature search on PubMed and Ovid across all years using the search terms identified in Table 1 was initially conducted in December 2020, with vaccine related articles extracted from this search. A Google search was also conducted to identify any grey literature relevant to our research questions. Following the initial search, a top-up search was conducted on PubMed, Ovid and Google using the top up search terms identified in Table 1 to identify any recently published peer-reviewed or grey literature, up to October 2021. The search strategy was reviewed to confirm the appropriateness of the search terms across databases.
Table 1.
Initial and subsequent search terms.
| Number | Search term |
|---|---|
| #1 | ((COVID-19) OR (SARS-Cov-2) OR (coronavirus)) |
| #2 | (vaccine) OR (vaccination) |
| #3 | ((cancer) OR (priority groups) OR (immunocompromised) OR (high-risk)) |
| #5 | ((#1) AND (#2) AND (#3)) |
| Top-up search | |
| #1 | ((COVID-19) OR (SARS-Cov-2) OR (coronavirus)) |
| #2 | (vaccine) OR (vaccination) |
| #3 | ((cancer) OR (priority groups) OR (immunocompromised) OR (high-risk)) |
| #4 | (hesitancy) OR (attitudes) OR (acceptance) |
| #5 | ((#1) AND (#2) AND (#3) AND (#4)) |
Articles were eligible for inclusion if they addressed rates of COVID-19 vaccination or vaccine hesitancy in cancer patients or Oncology professionals, or strategies to combat vaccine hesitancy in this population. Articles were required to be in English. Articles from any country or region were included. All study designs were eligible, however individual case studies, opinion pieces, and commentary articles were excluded. Identified title and abstracts were screened independently by two authors (RS and ST) using a standardised application of the eligibility criteria. Full text review was then conducted by one author (PB), with 55% checked by two others (JS and NB). All included and excluded articles were reviewed and any discrepancies were discussed amongst the review team until agreement was reached.
2.2. Data extraction
For studies meeting full inclusion criteria, data were extracted independently by one author (PB) on a standardised coding sheet, with 55 % checked for accuracy by another (NB). Discrepancies were raised with the review team with consensus reached through discussion. The following details were extracted: study title, authors, study design, study aim(s), sample size and setting, response rate, participants’ age and gender, details of any interventions implemented and comparison groups, and outcomes.
2.3. Assessment of study bias
Included studies were appraised for methodological quality by one author (PB), with 55 % cross-checked by two other authors (JS and NB). The Mixed-Methods Quality Appraisal Tool (MMAT) [12] was used to assess for potential bias, as it is applicable to all study types. The MMAT comprises two general yes/no questions, followed by five study-specific yes/no questions, resulting in a maximum score of 7. As the literature on this topic is new, no studies were excluded due to quality; results were, however, interpreted in light of quality. A summary is presented in Supplementary File 2.
2.4. Data synthesis
As studies were few and diverse methods and measures were reported, meta-analysis was not possible. A narrative synthesis was therefore undertaken, following BMJ guidelines [13]. Studies were sorted into those addressing each research question. For research questions 1–3, ranges of reported rates (percentages of populations studied) were determined for vaccine uptake and reported hesitancy. For research question 4, mean or median group differences, significance rates and effect sizes were collated for intervention studies. Qualitative studies were then examined for extracted themes, which were combined into meta-themes arising across studies. Consistency between quantitative and qualitative findings was explored, and potential reasons for inconsistency examined.
3. Results
The search yielded 18 articles which satisfied the eligibility criteria. Four were from USA, two from Poland, two from Mexico, two from Tunisia, one each from India, Hong Kong and Korea, one was international, and the remainder were from single European countries. Only one study [7] included Australian respondents, however the proportion of Australian respondents in that sample that were cancer patients cannot be determined. All but two were cross-sectional studies using study-developed measures; two were pre-post intervention studies (evaluating a webinar on the COVID-19 vaccine) [14], [15]. Sample sizes varied from 21,913 [7] to 50 [15], with most studies including people with heterogenous cancers and many recruiting from single institutions.
The included studies satisfied 0–100 % of quality criteria, with seven papers scoring 100 %, one paper 0 % and the remainder 60–80 %. The most common criteria not explicitly met were sample representativeness and potential bias. Results are presented by research question below.
-
1.
Are cancer patients getting vaccinated at the same rate as the general population?
We were unable to find any mature published data on how many cancer patients are receiving the COVID-19 vaccine in Australia or internationally, thus we were unable to compare their rates with that of the general population. Several included studies reported interim vaccination rates (ranging from 4.8 % in an Indian sample [16] to 71 % in a USA sample) [17], however as these were at varied stages of vaccination implementation, they do not represent final data.
-
2.
Is there hesitation among health professionals?
We have not located any literature that examines whether oncologists are hesitant in recommending a COVID-19 vaccine for their patients. Most articles assumed positive vaccine attitudes in health professionals. A majority of patients (79.1 %) in one study [18] said their physicians had recommended vaccination.
-
3.
Is there hesitation among patients – any types of cancer specifically?
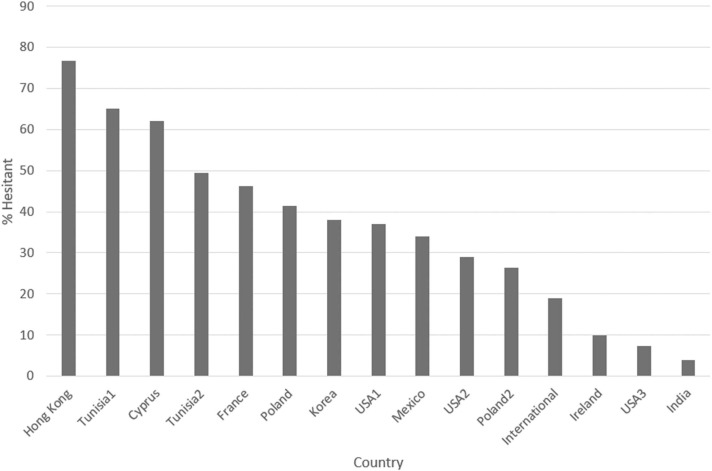
COVID-19 vaccine hesitancy rates in patients varied across studies from 82.1 % in Hong Kong [19] to 7.3 % in a US sample [17] with a mean of 38.4 % across studies (see Fig. 1). The large international study (n > 20,000) [7] reported a more conservative hesitancy rate of 19 %. A substantial number of hesitant patients were unsure or were waiting to see how other patients fared, before deciding. However, between 2 % in Ireland [20] and 45 % in Hong Kong [19] were definitely against or reluctant to accept vaccination (words used for similar concepts varied in different surveys).
Across studies, many factors were identified that were associated with COVID-19 vaccine hesitancy or reported by participants to influence their views on vaccination. A content analysis identified six main concerns, including about the:-
1)efficacy of the vaccine against COVID-19;
-
2)safety of the vaccine specifically for cancer patients (e.g. concerns about potential interactions with concomitant cancer treatments, reducing cancer treatment efficacy or increasing side-effects);
-
3)safety of the vaccine more generally (e.g. allergic reactions leading to death, impact on fertility or pregnancy, pain and fever);
-
4)effects of the vaccine on the timing (e.g. delays) and delivery of cancer treatment (e.g. avoiding treatments with large immunosuppressive effects);
-
5)scientific quality of the process and data underpinning the development of the vaccine; and
-
6)belief that COVID-19 is benign and will not lead to hospitalisation.
Less common concerns reported by < 2 % hesitant individuals were: a fear of needles, allergy to other vaccines, a fear of contracting COVID-19 from the vaccine, concern about payment for the vaccine, belief in vaccine dilution because of a limited supply, concern that the vaccine was made of cells from aborted fetuses, belief that the vaccine would change their DNA or carried a chip to enable surveillance of the population, belief that they did not need the vaccination because of their history of SARS-CoV-2 infection, and a preference to prevent with distancing and masking rather than vaccination.
Lack of faith in the government and/or media was reported to reduce willingness to vaccinate, playing a significant role in Tunisian patients’ acceptance of the vaccine [21]. In this study patients not registered (OR = 5.9, 95 %CI [1.58–8.7]) or not informed about the national vaccination programme (OR = 5.51, 95 %CI [2.1–7.9]) were more likely to be against vaccination. In a large international study [7], more conservative political views (political views aligned with small government and more traditional social values) were associated with hesitancy, while in the USA, States with a majority of Republican voters have seen lower rates of vaccination.
Several studies reported that people with a higher level of health literacy [19], [22]. or education [17] were less hesitant, perhaps because they were better able to access and understand the science behind vaccination.
Chun et al [23]. found demographic characteristics (male gender, older age, influenza vaccination history) were associated with higher vaccination rates in their Korean sample, perhaps because these groups felt more at risk of severe COVID-19 disease. Similarly, in the US [8] older, white, male or urban participants were more willing to vaccinate. The larger international study [7] reported that younger female patients were more hesitant, perhaps because of fertility/pregnancy concerns.
Chun et al [23]. also found disease characteristics (absence of cancer recurrence, time since cancer diagnosis over 5 years, and higher quality of life scores) were associated with higher acceptance rates of vaccination. Better performance status was also associated with greater willingness to vaccinate in an Italian sample [24], suggesting that those less impacted by cancer were more able to focus on vaccination or were less concerned about cancer interactions. Indeed, participants in another study [21] who thought the vaccine may interfere with treatment efficacy (OR = 7.28, 95 %CI [2.5–12.32]), or would impact cancer outcomes (OR = 6.14, 95 %CI [2.27–16.7]), were significantly more likely to refuse a vaccine.
-
1)
-
4.
Is there any evidence regarding strategies to address this vaccine hesitancy?
Government policies and communication were found to impact vaccination. Several studies noted changes in attitudes after key government announcements (e.g., regarding limiting access (or not) of some groups to the AstraZeneca vaccine due to rare side-effects). Choice of vaccine also impacted hesitancy with more patients willing to be vaccinated after the Pfizer vaccine was made available [25]. In one study, hesitant patients endorsed arguments that could convince them to proceed, including: more information on efficacy (59.4 %), safety (50.3 %), type of vaccine administered (35.2 %), duty for collective responsibility or return to normalcy (7.4 %) [18]. A substantial minority (31.6–45.7 %) of patients in several studies [18], [21] supported mandatory vaccination.
Fig. 1.
Vaccine hesitancy rates (undecided or against) reported across studies. *Vaccine hesitancy rates were reported for multiple separate cohorts from Tunisia, the USA, and Poland which are reflected in the numbering.
Many patients felt inadequately informed about the vaccines, with several studies documenting low vaccine literacy [22], [23]. One study [19] reported that 45 % of participants responded ‘don’t know’ to all five knowledge questions posed. Those with more information about vaccine efficacy were found to be less hesitant [16], and when asked what would convince them to be vaccinated, 30.3 % of participants in another study endorsed information and education [16]. Two small pre-post intervention studies investigated the utility of a webinar for oncology patients providing information and endorsing vaccination. In the first [15], significantly reduced hesitation was observed post-webinar. Of the 11 people who completed both surveys, significantly more participants acknowledged COVID-19 vaccines were safe (45 % v 100 %, p = 0.031), effective (36 % v 91 %, p = 0.031), and recommended (45 % v 100 %, p = 0.031) for cancer patients, post -webinar. However, in the second larger study [14] of 105 patients who completed pre and post webinar surveys, only three people shifted towards more positive vaccination views post-webinar.
Many studies reported patients were seeking expert, personalised information from their oncologist [26], and were readily persuaded after a short positive conversation with their oncologist; willingness to vaccinate was significantly associated with having spoken to an oncology health professional about vaccination (p < 0.0001) [16], or being recommended by their oncologist to be vaccinated (OR, 3.29; 95 % CI, 2.27–4.77) [15]. However, in one study [27] while the majority of patients intending to be vaccinated considered oncologists qualified to advise patients on vaccination, patients not intending to be vaccinated considered personal judgement the main source of reliable information; thus, oncologist recommendation may be ineffective for a subset of patients with strong anti-vaccination views. Another study [14] tested framing approaches (positive focusing on efficacy, negative focusing on failure rate) and found that more participants said they would have the vaccine in the positive framing condition.
Finally, one Indian study [16] reported a small number of patients (2.6 % of the sample) said an improvement in their general wellbeing or return to their hometown would convince them to be vaccinated. As depression was found to be associated with hesitancy in another study [19], this suggests that addressing quality of life and well-being may decrease hesitancy overall.
4. Discussion and conclusions
4.1. Discussion
This review provides a synthesis of evidence regarding COVID-19 vaccination in oncology patients, identifying 18 articles addressing this issue, all published in 2021. We found limited evidence on vaccination uptake in oncology patients, thus comparisons of oncology patients with general populations were not possible. While population-based data collection linking vaccination to cancer status may be a future strategy, at this stage, collection of COVID-19 vaccination status in oncology patients at the local institution level is most likely to yield accurate data quickly.
While data on actual vaccination rates was limited, most studies reported rates of hesitancy, with figures widely ranging from 82.1 % in a Hong Kong sample [19] to 7.3 % in a US sample [17]. Across studies, a mean of 38.4 % of participants reported hesitancy, although the largest study by far, with over 20,000 participants from international sources [7] reported a more conservative hesitancy rate of 19 %. Of the hesitant groups, between 2 % [20] and 45 % [19] were definitely against or reluctant to accept vaccination, thus likely to be in the resistant minority who will remain unvaccinated. Acknowledging that hesitancy will likely be impacted by pandemic severity, vaccine safety and efficacy data, government policies and a host of other factors, these data suggest that hesitancy remains an important issue likely to impact outcomes for cancer patients, that will continue to require attention during this and future pandemics.
As questions were worded somewhat differently in the study-developed surveys utilised and vaccination accessibility varied across countries, conclusions about national differences in COVID-19 vaccination hesitancy rates can be made only with caution. However, what is clear from the included studies, is that individual government policies and communications impact cancer patients’ views on vaccination. Furthermore, trust in the government both more broadly and specifically in relation to handling the pandemic, are important in generating trust in vaccination.
This review identified a wide range of factors commonly impacting hesitancy in cancer patients. While many of these factors have been reported in surveys of the general population, several were specific to the cancer context. Cancer patients were quite reasonably concerned about potential interactions with concomitant cancer treatments, reducing cancer treatment efficacy or increasing side-effects, and about the effects of the vaccine on the timing and delivery of cancer treatments. For example, they were concerned their cancer treatment may be delayed after vaccination, that treatment side-effects would be exacerbated or confused with vaccination side-effects, or that treatments potentially more effective but with immunosuppressive effects might be withheld. Patients with poor health literacy or low education were more hesitant [15], suggesting a need for varied information approaches to address misinformation. Interventions such as public forums and focus groups, informational pamphlets in clinics and peer-to-peer counselling may increase vaccine acceptancy in people with cancer. Addressing social media may also be required, since many vaccine-hesitant participants rely primarily on this information source [28].
Patients looked to their oncologists to provide personalised information about vaccination which took into account their cancer situation. While data are limited on some of these questions, oncologists could assist by acknowledging these uncertainties, making cancer centre policies transparent, and discussing vaccination openly with their patients. Furthermore, as patients with better quality of life and mental state were more open to vaccination [16], [24], optimising supportive care is likely to be helpful, particularly as lockdown and social distancing policies have impacted the support available to patients [29] (acknowledging that the resources of many oncology services have been stretched to the limit during the pandemic).
We identified almost no information about attitudes and behaviours of oncologists regarding vaccination. Internationally, many national vaccination and oncology organisations have recommended COVID-19 vaccination for people with cancer, including those who are immunocompromised, since the benefits of vaccination are considered to outweigh any potential risks. These include bodies such as the Medical Oncology Group of Australia and the Australian Technical Advisory Group on Immunisation, the US National Comprehensive Cancer Network and the European Society for Medical Oncology [30], [31], [32], [33]. However, the decision about whether patients receive a COVID-19 vaccine should be made on an individual basis by the person affected by cancer, in consultation with their healthcare team. Thus, further research is required to better understand the attitudes of oncologists towards vaccination, and the approaches they use when discussing COVID-19 vaccination with their patients.
Limitations of this review include the recency and urgency with which this literature has emerged. Most studies were cross-sectional, and utilised study-developed measures. We utilised rapid review methods, and a systematic review and meta-analysis may be helpful in the future as the literature develops.
4.2. Conclusions
In conclusion, COVID-19 vaccine hesitancy rates in cancer patients vary widely across studies but remain high, with on average one in five patients experiencing hesitancy and most reporting concerns related to interactions between vaccines and the cancer context.
4.3. Practice implications
Oncologists appear key in providing personalised, tailored information to cancer patients about vaccination. Tailored education programmes and communication skills training for oncologists are likely to be required to maximise the quality and utility of these discussions. In a recent qualitative study, many oncologists reported a need for information and support when discussing COVID-19 with their patients [34]. While there is little evidence specifically within the cancer context to guide the content of this training, one study [15] has suggested that positively framing the effectiveness of vaccination in preventing COVID-19 infection, reducing severity of disease and reducing transmission is more effective than negative framing (failure rate). Furthermore, the larger vaccination literature contains many useful evidence-based strategies for vaccination discussions which may be useful in this context [35].
Webinars for cancer patients and peer-to-peer counselling may be useful strategies but remain to be proven.
CRediT authorship contribution statement
Phyllis Butow contributed to the conceptualisation of the review, led the formal analysis, wrote the initial draft of the paper and prepared the final paper. Joanne Shaw contributed to the conceptualisation of the review. contributed to formal analysis, and reviewed and commented on successive paper drafts. Nicole Bartley contributed to the formal analysis, and reviewed and commented on successive paper drafts. Vivienne Milch contributed to the conceptualisation of the review, facilitated data-base searches, and reviewed and commented on successive paper drafts. Rahul Sathiaraj contributed to the conceptualisation of the review, conducted data-base searches, and reviewed and commented on successive paper drafts. Scott Turnbull contributed to the conceptualisation of the review, conducted data-base searches, and reviewed and commented on successive paper drafts. Carolyn Vartanian contributed to the conceptualisation of the review, facilitated data-base searches, and reviewed and commented on successive paper drafts.
Declaration of Interests Form
The authors have no interests to declare regarding the paper below:
Vaccine hesitancy in cancer patients: A Rapid Review.
Phyllis Butow, Joanne Shaw, Nicole Bartley, Vivienne Milch, Rahul Sathiaraj, Scott Turnbull, Carolyn Vartanian.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pec.2023.107680.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.World Health Organisation. 〈https://covid19.who.int〉.
- 2.Carreira H., Strongman H., Peppa M., McDonald H.I., Dos-Santos-Silva I., Stanway S., et al. Prevalence of COVID-19-related risk factors and risk of severe influenza outcomes in cancer survivors: a matched cohort study using linked English electronic health records data. Lancet. 2020;29 doi: 10.1016/j.eclinm.2020.100656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuderer N.M., Choueiri T.K., Shah D.P., Shyr Y., Rubinstein S.M., Rivera D.R., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee L.Y.W., Cazier J.B., Starkey T., Briggs S.E.W., Arnold R., Bisht V., et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21:1309–1316. doi: 10.1016/S1470-2045(20)30442-3. [doi:10.1016/] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.Cd-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai R., Hervey J., Hoffman K.D., Wood J., Novack J., Johnson J., et al. COVID-19 hesitancy among individuals with cancer, autoimmune diseases, and other serious comorbid conditions. medRxiv. 2021 doi: 10.1101/2021.04.06.21254014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti R, Akesson J, Weiss E, Sae-Hau M, Lee M, Gracia G , et al. COVID-19 vaccine hesitancy among blood cancer patients. 〈https://www.lls.org/research/covid-19-vaccine-hesitancy-among-blood-cancer-patients〉.
- 9.Kufel-Grabowska J., Bartoszkiewicz M., Ramlau R., Litwiniuk M. Cancer patients and internal medicine patients attitude towards COVID-19 vaccination in Poland. Adv Clin Exp Med. 2021;30(8):805–811. doi: 10.17219/acem/138962. . PMID: 34286517. [DOI] [PubMed] [Google Scholar]
- 10.Hamel C., Michaud A., Thuku M., Skidmore B., Stevens A., Nussbaumer-Streit B., et al. Defining rapid reviews: a systematic scoping review and thematic analysis of definitions and defining characteristics of rapid reviews. J Clin Epidemiol. 2021;129:74–85. doi: 10.1016/j.jclinepi.2020.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Garritty C, Gartlehner G, Kamel C, King VJ, Nussbaumer-Streit B, Stevens A , et al. Cochrane rapid reviews. Interim Guidance from the Cochrane Rapid Reviews Methods Group; 2020. [DOI] [PMC free article] [PubMed]
- 12.Bartlett G., Vedel I., Hong Q.N., et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf. 2018;34:285–291. doi: 10.3233/EFI-180221. [Article] [DOI] [Google Scholar]
- 13.Campbell M., McKenzie J.E., Sowden A., Katikireddi S.V., Brennan S.E., Ellis S., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelkar A.H., Blake J.A., Cherabuddi K., Cornett H., McKee B.L., Cogle C.R. Vaccine enthusiasm and hesitancy in cancer patients and the impact of a webinar. Healthcare. 2021;9(3):351. doi: 10.3390/healthcare9030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villareal-Garza C., Vaca-Cartagena B.F., Becerril-Gaitan A., Castilleja-Leal F. Letter to editor, Re: The first report on Covid-19 vaccine refusal by cancer patients in Italy: early data from a single-institute survey. Educational Webinar about COVID-19 vaccines in oncological patients: a promising strategy to tackle COVID-19 vaccine hesitancy. Eur J Cancer. 2021;158:189–190. doi: 10.1016/j.ejca.2021.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noronha V., Abraham G., Bondili S.K., Raipurohit A., Menon R.P., Gattain S., et al. COVID-19 vaccine uptake and vaccine hesitancy in Indian patients with cancer: a questionnaire-based survey. Cancer Res Stat Treat. 2021;4:211–218. 〈https://www.crstonline.com/text.asp?2021/4/2/211/320134〉 [Google Scholar]
- 17.Figueiredo J.C., Ihenacho U., Merin N.M., Stewart J.L., Merchant A., Reckamp K.L., et al. SARS-CoV-2 vaccine uptake, perspectives, and adverse reactions following vaccination in patients with cancer undergoing treatment. Ann Oncol. 2021 doi: 10.1016/j.annonc.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brodziak A., Sigorski D., Osmola M., Wilk M., Gawlik-Urban A., Kiszka J., et al. Attitudes of patients with cancer towards vaccinations – results of online survey with special focus on the vaccination against COVID-19. Vaccines. 2021;9(5):411. doi: 10.3390/vaccines9050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan W.L., Ho Y.T., Wong C.K., Choi H.C., Lam K.O., Yuen K.K., et al. Acceptance of COVID-19 vaccination in cancer patients in Hong Kong: approaches to improve the vaccination rate. Vaccines. 2021;9(7):792. doi: 10.3390/vaccines9070792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullaly W.J., Flynn C., Carr P., Grant C., Kelleher F. Acceptance of COVID-19 vaccination among cancer patients in an Irish cancer centre. Ann Oncol. 2021;32(S5):S1144–S1145. doi: 10.1016/j.annonc.2021.08.1588. [DOI] [Google Scholar]
- 21.Mejri N., Berrazega Y., Ouertani E., Rachdi H., Bohli M., Kochbati L., et al. Understanding COVID-19 vaccine hesitancy and resistance: another challenge in cancer patients. Support Care Cancer. 2022;30(1):289–293. doi: 10.1007/s00520-021-06419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherif I., Khiari H., M'ghirbi F., Mallekh R., Mezlini A., Hsairi M. COVID-19 vaccine hesitancy among Tunisian cancer patients in the Salah Azaeiz Institute of Cancer. Eur J Public Health. 2021;31(S3) doi: 10.1093/eurpub/ckab165.056. [DOI] [Google Scholar]
- 23.Chun J.Y., Kim S.I., Park E.Y., Park S.Y., Koh S.J., Cha Y., et al. Cancer patients' willingness to take COVID-19 vaccination: a nationwide multicenter survey in Korea. Cancers. 2021;13(15):3883. doi: 10.3390/cancers13153883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Noia V., Renna D., Barberi V., Di Civita M., Riva F., Costantini G., et al. The first report on coronavirus disease 2019 (COVID-19) vaccine refusal by patients with solid cancer in Italy: early data from a single-institute survey. Eur J Cancer. 2021;153:260–264. doi: 10.1016/j.ejca.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waters A.R., Kepka D., Ramsay J.M., Mann K., Lopez P.L.V., Anderson J.S., et al. COVID-19 vaccine hesitancy among adolescent and young adult cancer survivors. JNCI Cancer Spectr. 2021;5(3):pkab049. doi: 10.1093/jncics/pkab049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villarreal-Garza C., Vaca-Cartagena B.F., Becerril-Gaitan A., et al. Attitudes and factors associated with COVID-19 vaccine hesitancy among patients with breast cancer. JAMA Oncol. 2021;7(8):1242–1244. doi: 10.1001/jamaoncol.2021.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrière J., Gal J., Hoch B., Cassuto O., Leysalle A., Chamorey E., et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32(5):673–674. doi: 10.1016/j.annonc.2021.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roupa Z., Noula M., Nikitara M., Ghobrial S., Latzourakis E., Polychronis G. Acceptance of coronavirus disease 2019 vaccination by cancer patients in Cyprus: a cross-sectional study. J Oncol Pharm Pract. 2021 doi: 10.1177/10781552211039489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell A.J., Chan M., Bhatti H., Halton M., Grassi L., Johansen C., et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi: 10.1016/S1470-2045(11)70002-X. [DOI] [PubMed] [Google Scholar]
- 30.Medical Oncology Group of Australia. COVID-19 vaccination in patients with solid tumours. Medical Oncology Group of Australia; 2021 [updated 17/10/2021. Available from: 〈https://www.moga.org.au/all-position-statements/covid-19-vaccination-in-patients-with-solid-tumours〉. [DOI] [PMC free article] [PubMed]
- 31.Australian Technical Advisory Group on Immunisation. Provider guide to COVID-19 vaccination of people with immunocompromise. Australian Government, Department of Health; 2021 [updated 29/10/2021. Available from: 〈https://www.health.gov.au/sites/default/files/documents/2021/08/atagi-provider-guide-to-covid-19-vaccination-of-people-with-immunocompromise.pdf〉.
- 32.National Comprehensive Cancer Network. National comprehensive cancer network: cancer and COVID-19 vaccination. National Comprehensive Cancer Network; 2021 [updated 30/08/2021. Available from: 〈https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v4-0.pdf?sfvrsn=b483da2b_70〉.
- 33.European Society for Medical Oncology. COVID-19 vaccination in patients with cancer: ESMO releases ten statements. European Society for Medical Oncology; 2020 [updated 22/12/2020. Available from: 〈https://perspectives.esmo.org/news/covid-19-vaccination-in-patients-with-cancer-esmo-releases-ten-statements〉.
- 34.Butow P, Havard PE, Butt Z, Juraskova I, Sharpe L, Dhillon H , et al. Stakeholder perspectives on the impact of Covid-19 on oncology services: a qualitative study. Clinical Oncology Society of Australia virtual meeting; 2021. [DOI] [PMC free article] [PubMed]
- 35.Leask J., Carlson S.J., Attwell K., Clark K.K., Kaufman J., Hughes C., et al. Communicating with patients and the public about COVID-19 vaccine safety: recommendations from the Collaboration on Social Science in Immunisation. MJA. 2021;215(1):9–12. doi: 10.5694/mja2.51136. [e1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material