FIGURE 1.
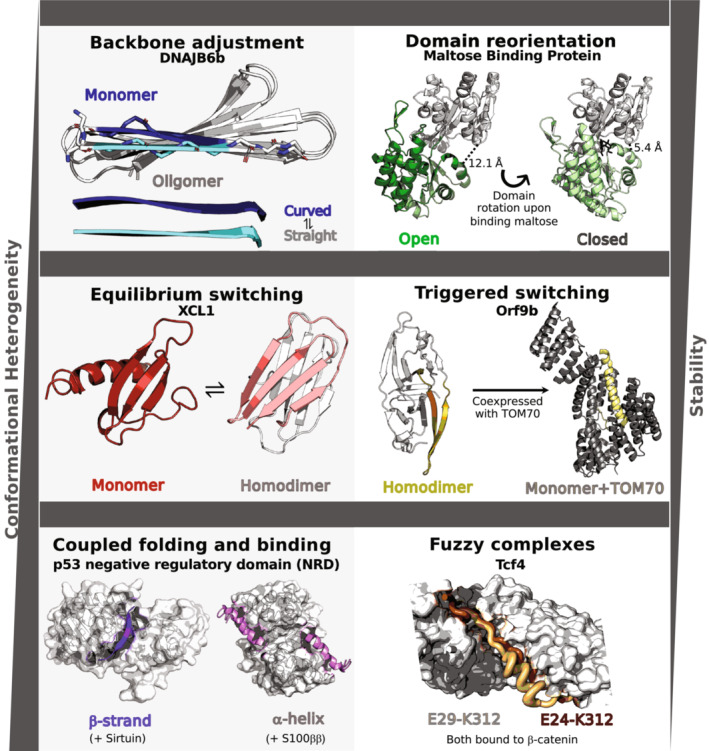
Increasing conformational heterogeneity and decreasing stability are generally observed among single‐fold, fold‐switching, and intrinsically disordered proteins, respectively. Examples of single‐fold proteins include backbone adjustment and domain reorientation. The former is illustrated by DNAJB6b, a chaperone whose N‐terminal β‐strand is twisted in its monomeric configuration (dark blue, PDB ID: 7JSQ, model 1) but straight in oligomers (cyan, PDB ID: 6U3R, Model 1); C‐terminal β‐sheets are light gray. The orientation of Maltose Binding Protein's N‐terminal (light gray) and C‐terminal domains differ in the open apo (dark green, PDB ID: 1JW4, Chain A) and close maltose‐bound (light green, 1ANF, Chain A) forms. Interdomain distances in both conformations are annotated. Structural heterogeneity in fold‐switching proteins can be categorized into equilibrium switching, in which both conformations are populated at detectable levels under certain conditions, and triggered switching, in which an external stimulus shifts the protein from one dominant fold to another. Equilibrium switching is exemplified by XCL1, a human chemokine that populates a monomeric α + β fold (red, PDB ID: 1J9O, Model 1) and a dimeric β‐sheet fold with a completely different hydrogen bond network (one unit salmon, the other light gray, PDB ID: 2 N54, Model 1) at about a 50:50 ratio under physiological conditions. Orf9b illustrates triggered switching; expressed in isolation, this severe acute respiratory syndrome coronavirus‐2 protein folds into a β‐sheet homodimer (olive, PDB ID: 6Z4U, Chains A and B), while a portion of it folds into an α‐helix (yellow, PDB ID: 7DHG, Chain B) when coexpressed with human TOM70 (light gray, PDB ID: 7DHG, Chain A), which it binds in situ. Intrinsically disordered proteins, which display the most structural heterogeneity, can fold into different secondary structures upon binding different partners (Coupled folding and binding) and upon binding the same partner (Fuzzy complex). Human tumor suppressor protein p53 demonstrates coupled folding and binding both by folding into a β‐sheet (purple, PDB ID: 1MA3, Chain B) when complexed with Sirtuin (gray surface, PDB ID: 1MA3, Chain A) and by folding into an α‐helix (pink, PDB ID: 1DT7, Chains X and Y) when complexed with S100ββ (gray surface, PDB ID: 1DT7, Chains A and B). Finally, Tcf4 forms a fuzzy complex with β‐catenin (gray surface, PDB ID: 1G3J, Chain A); two distinct bound conformations are shown in light orange (PDB ID: 1JDH, Chain B) and brown (PDB ID: 1G3J, Chain B). This brown conformation is a proposed model only as it shows the conformation of a highly similar homolog, Tcf3. Mutagenesis experiments demonstrate that Tcf4 can interact with β‐catenin in manners consistent with both structures.
