Abstract
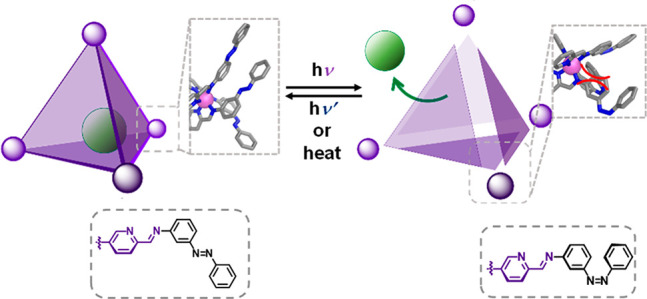
A strategy for light-powered guest release from a tetrahedral capsule has been developed by incorporating azobenzene units at its vertices. A new Zn4L4 tetrahedral capsule bearing 12 diazo moieties at its metal-ion vertices was prepared from a phenyldiazenyl-functionalized subcomponent and a central trialdehyde panel. Ultraviolet irradiation caused isomerization of the peripheral diazo groups from the thermodynamically preferred trans configuration to the cis form, thereby generating steric clash and resulting in cage disassembly and concomitant guest release. Visible-light irradiation drove cage re-assembly following re-isomerization of the diazo groups to the trans form, resulting in guest re-uptake. A detailed 19F NMR study elucidated how switching led to guest release: each metal vertex tolerated only one cis-azobenzene moiety, with further isomerization leading to cage disassembly.
Discrete supramolecular architectures1 and containers2 can undergo structural re-configuration upon receiving external stimuli, which can generate useful functions.3 Stimuli-responsive guest uptake and release by supramolecular capsules4 is one such function, potentially enabling new means of drug delivery,5 pesticide release,6 chemical separations,7 and purifications.8 Among stimuli,9 light is particularly useful, as it is easy to apply from cheap sources and does not result in the accumulation of waste products even after multiple cycles.10
Photoswitches have been used to control encapsulation and release processes.11 For example, Clever and co-workers recently prepared light-responsive coordination cages based upon dithienylethene12 and diazocine13 chromophores, which can undergo structure transformations that prompt guest uptake and release. The Fujita group reported photoisomerization of inward-facing azobenzene moieties in a spherical complex, where switching resulted in reversible guest uptake.14 In these systems, the photochromic moieties form integral parts of the ligand backbone, necessitating a re-design of the cage to target new guests. A more general and modular approach would decouple the photochrome from the cage framework, to render guest-binding orthogonal to the photoswitching process that governs guest uptake and release.
Subcomponent self-assembly provides a modular construction technique for metal–organic capsules that respond to different stimuli.15 Systems have thus been designed using acid and base as stimuli to release and exchange cargos between two capsules, through the selective disassembly of one.16 Systems containing two or three capsules have been reported, where selective disassembly of individual cages and the release of their guests were guided by the application of external signals.17 In each case, hosts disassemble following the addition of chemical signals, resulting in the accumulation of chemical waste products after each addition. The use of light or heat18 as a stimulus would avoid the formation of such byproducts, sidestepping problems associated with waste buildup.
Azobenzenes serve as key photochromic moieties within supramolecular assemblies, as they not only alter dipole moment14 but also change shape to alter properties in useful ways.19 Building upon reports of photoinduced guest uptake and release from multilayer films20 and nanotubes,21 herein we report a discrete and solution-based22 self-assembled Zn4L4 tetrahedral cage, trans-1 (Figure 1), functionalized with 12 azobenzene units at its vertices. The photoswitching of these azobenzenes toward the cis configuration results in progressive buildup of steric strain, culminating in the release of an anionic guest bound within the cage cavity.
Figure 1.

a) Assembly of subcomponents A and trans-B with ZnII produced cage trans-1. b) 1H and DOSY NMR (400 MHz, CD3CN, 298 K) spectra of trans-1.
Photoswitchable azobenzene-functionalized subcomponent 3-(phenyldiazenyl)aniline B was synthesized following a literature procedure.23B forms as the trans isomer (trans-B), which is stable in the absence of light. Prior to investigating cage photoisomerization, we first tested the photoswitching ability of subcomponent trans-B. Irradiation at 350 nm generates the cis isomer (cis-B), while irradiation at 500 nm or heating reverses the process (Figure S20). Upon exposure to UV light, a new set of signals appeared in the 1H NMR spectrum, assigned to cis-B. The isomerization did not occur quantitatively. A photostationary state (PSS) was reached after 10 min and determined to consist of 71% cis-B by NMR integration (Figure S21). Upon irradiation at 500 nm, trans-B was fully regenerated within 30 min; complete conversion to trans-B also occurred after heating the PSS to 75 °C for 600 min, revealing a half-life of 46 min at that temperature (Figures S22 and S23).
Tritopic formylpyridine subcomponent A was prepared from 2,4,6-trichloro-1,3,5-triazine and 5-hydroxypicolinaldehyde (see Supporting Information Section S2). Subcomponents trans-B (12 equiv) and A (4 equiv) reacted with zinc(II) bis(trifluoromethanesulfonyl)imide (triflimide, Tf2N–, 4 equiv) in acetonitrile to give tetrahedral capsule trans-1 (Figure 1a) as the uniquely observed product. Trans-1 was characterized by 1H NMR, 1H–1H COSY, DOSY, and electrospray ionization mass spectroscopy (ESI-MS) (Figures S3–S10). The 1H NMR spectra of trans-1 exhibited only one set of ligand signals, consistent with a T-symmetric tetrahedral product (Figure 1b).24 A 1H DOSY spectrum of the cage corroborates the presence of a single component with a diffusion coefficient (D) of 3.9 × 10–10 m2·s–1 (Figure 1b). We infer that the three trans-diazo moieties surrounding each ZnII center of trans-1 extend from each other without steric collisions (Figure 2b).
Figure 2.

(a) Crystal structure of Tf2N–⊂2. Tf2N– shown in space-filling mode: N, blue; S, yellow; O, red; C, gray; F, pale green. Disorder, unbound counterions, and solvent of crystallization are omitted for clarity. Attempts to obtain single crystals suitable for X-ray diffraction of an analog of cage 2 with ZnII instead of FeII were unsuccessful. (b) MM2-optimized molecular model of trans-1, based on the structure of 2. (c) 19F NMR (376 MHz, CD3CN, 298 K) spectrum of Tf2N–⊂trans-1.
Although multiple attempts to crystallize cage trans-1 were unsuccessful, single crystals suitable for X-ray diffraction of analogous cage 2, composed of subcomponent A, iron(II) triflimide, and p-toluidine in place of B, were obtained following slow diffusion of diethyl ether into a solution of 2 in acetonitrile. As shown in Figure 2a, Tf2N– was found inside the 205 Å3 cavity of cage 2. Furthermore, the 1H NMR and 19F NMR spectra of cage 2 show two sets of signals for the empty and triflimide-binding cage in slow exchange on both NMR time scales (Figures S15 and S16).
The 19F NMR spectrum (Figure 2c) of cage trans-1 displayed two sets of signals for the Tf2N– counter anion, in a 7:1 integral ratio. This observation is consistent with one anion being bound within the cavity of 1 (Figure 2b), as observed in the crystal structure of 2 (Figure 2a), with the other seven free in solution.
Since attempts to encapsulate neutral guests by trans-1 provided no 1H NMR evidence of binding (Figure S57), subsequent host–guest studies were conducted with anions only. PF6–, TfO–, and BF4– were observed to bind within trans-1 (Figure S47). Progressive addition of the tetrabutyl ammonium salt of each anion to a solution of Tf2N–⊂trans-1 showed evidence of triflimide displacement by 1H NMR (Figures S37, S39, and S43) and 19F NMR (Figures S38, S40, and S44). In each case, the 19F NMR resonances for the guest molecules were broadened and shifted, suggesting fast-exchange binding of anions within trans-1 on the NMR time scale even at 233 K (Figures S51–S55). We attribute the observed differences in exchange properties to the smaller volumes of PF6–, TfO–, and BF4–, as compared to Tf2N– (Table S1). Moreover, attempts to prepare the cage with Zn(OTf)2 and Zn(BF4)2 instead of Zn(NTf2)2 appeared successful by 1H NMR (Figures S11 and S13). Studies involving these anions were not pursued further because slow-exchanging triflimide greatly facilitated analysis by NMR (see below).
We then investigated light-driven guest release for Tf2N–⊂trans-1 (Figure 3). Upon irradiation with 350 nm light, the trans-azobenzene subcomponents at the periphery of trans-1 underwent photoisomerization to the cis isomer. This photochemical transformation was followed by 1H NMR and 19F NMR. The 1H NMR spectra became complex, with multiple sets of ligand signals, suggesting the formation of a mixture of cages containing both cis- and trans-diazo moieties (Figure S26).
Figure 3.
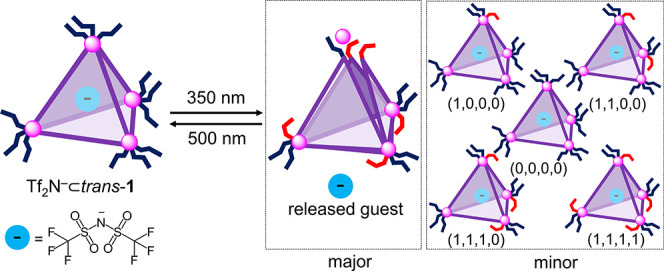
Cartoon of the photoswitching of cage Tf2N–⊂trans-1, illustrating the opening of the cage after a fifth azobenzene switches, along with the five observed guest-binding states of the cage, in which 0–4 azobenzene residues have switched to cis. B residues with the trans and cis configurations are colored blue and red, respectively.
Encouragingly, photoisomerization led to the partial disassembly of the cage, with appearance of 1H NMR signals for subcomponents A and cis-B (Figure S27). We infer this disassembly to have occurred as a result of cis-azobenzenes occupying more of the volume near the metal center than in the case of the trans isomer, leading to steric collisions between subcomponents at the metal-ion vertices of the cage. When the disassembled system was irradiated at 500 nm, the azo moieties converted back to the trans configuration and re-assembled to form Tf2N–⊂trans-1, as confirmed by 1H NMR (Figure S28).
The photoisomerization abilities of B were thus maintained following integration into the metallocage framework of 1. Upon removal of the light source and subsequent heating to 75 °C, the disassembled system started transforming into Tf2N–⊂trans-1 with time (Figure S29). The process was monitored by 19F NMR, revealing a half-life of 38 min (Figure S32), similar to that of free subcomponent photoisomerization. After 600 min at 75 °C, the spectrum was identical to that of freshly prepared Tf2N–⊂trans-1.
The 19F NMR spectra of Tf2N–⊂trans-1 during irradiation were more straightforward to interpret than the corresponding 1H NMR spectra, allowing the course of cage isomerization to be followed. As noted above, prior to UV irradiation, only one 19F NMR signal was observed for the encapsulated Tf2N– guest. After UV irradiation, however, five distinct bound Tf2N– signals were observed (Figure 4). We infer that these species incorporated from 0 to 4 cis-diazo moieties per cage (Figure 3), with each cage tolerating a maximum of one cis-diazo per vertex before opening to release the guest (Figure S71). Evolution of the 19F NMR signals (Figure S30) is consistent with the most upfield peak corresponding to cages with 4 cis-diazo moieties per cage, whereas the most downfield peak corresponds to trans-1, i.e., a cage without cis-diazo moieties (Figure 4). Analysis of the extent of progressive photoisomerization in cage 1 is difficult to quantify because the triflimide 19F NMR signal reports only on intact cages, with no straightforward means available to quantify the cis vs trans population of the ligand that is no longer incorporated into a cage (Figure S34). Figure 5 shows the time course of the reverse of this process, as free Tf2N– is encapsulated by cages that incorporate progressively more trans residues as the system thermally relaxes from a state in which most B residues are cis.
Figure 4.

19F NMR spectra (CD3CN, 376 MHz, 298 K) show 43% encapsulated/57% released Tf2N– guest after UV irradiation for 10 min; the encapsulated Tf2N– is quantified by NMR integration with respect to hexafluorobenzene as an internal standard. The five distinct guest-encapsulating cages, incorporating 0–4 cis-azobenzene residues, gave rise to five 19F NMR signals, which were deconvoluted as shown.
Figure 5.

Re-formation of cages incorporating progressively more residues of trans-B during thermal relaxation while heating to 75 °C, derived from integration of 19F NMR spectra of the encapsulated triflimide guest (Supporting Information, Figure S30). The percentage population value shown is normalized to the maximum amount of anion that can be encapsulated.
We then quantified the release process by using hexafluorobenzene as an internal standard (Figure 4). The integration of 19F NMR signals revealed 57% release of the Tf2N– guest 10 min after irradiation (Figure S33). The system showed no evidence of fatigue after 10 cycles of alternating irradiation with 350 and 500 nm light (Figure 6b). Thermal recovery at 75 °C (Figure 6c) also proved entirely reversible, although with a longer cycle time of 600 min (Figure S31) vs 30 min for the purely light-driven process of Figure 6b. The light-triggered release and uptake of the other anionic guests, PF6–, TfO–, and BF4–, was also explored and confirmed by 1H and 19F NMR analyses (Figures S60–S69).
Figure 6.

a) Cartoon representation of reversible release and uptake of Tf2N– guest by UV light and visible light or heat. b) Ten cycles of guest release driven by irradiation at 350 and 500 nm in CD3CN in an alternating sequence. c) Three such cycles using light (350 nm) and temperature (75 °C) as stimuli; in both cases no evidence of fatigue was observed.
Although the cage 1 can only encapsulate non-biologically relevant anions, larger cages constructed from polytopic aldehyde subcomponents25 may enable this modular and straightforward means of light-powered guest release and uptake to find applications across different fields, potentially including switchable catalysis, drug delivery, and chemical purification.
In this latter application, a cargo molecule might be selectively bound and then released by light following flow to a different location. The ability to use light as a stimulus may also allow for stimulus transduction, where illumination releases guests as signals within more complex chemical systems.26
Acknowledgments
This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC) (EP/T031603/1) and the European Research Council (ERC 695009). The authors thank the Department of Chemistry NMR facility at the University of Cambridge for performing some NMR experiments. A.G. acknowledges the Deutsche Forschungsgemeinschaft for a Walter Benjamin postdoctoral fellowship. L.K.S.v.K. acknowledges the Alexander von Humboldt Foundation for a Feodor Lynen Research Fellowship. We also thank the Diamond Light Source (UK) for synchrotron beamtime on I19 (MT7569).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.2c10084.
Complete experimental details and characterization data (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Kundu S.; Ghosh A.; Paul I.; Schmittel M. Multicomponent pseudorotaxane quadrilateral as dual-way logic AND gate with two catalytic outputs. J. Am. Chem. Soc. 2022, 144, 13039–13043. 10.1021/jacs.2c05065. [DOI] [PubMed] [Google Scholar]; b Jellen M. J.; Liepuoniute I.; Jin M.; Jones C. G.; Yang S.; Jiang X.; Nelson H. M.; Houk K. N.; Garcia-Garibay M. A. Enhanced gearing fidelity achieved through macrocyclization of a solvated molecular spur gear. J. Am. Chem. Soc. 2021, 143, 7740–774. 10.1021/jacs.1c01885. [DOI] [PubMed] [Google Scholar]; c Wang J.; Zhao H.; Chen M.; Jiang Z.; Wang F.; Wang G.; Li K.; Zhang Z.; Liu D.; Jiang Z.; Wang P. Construction of macromolecular pinwheels using predesigned metalloligands. J. Am. Chem. Soc. 2020, 142, 21691–21701. 10.1021/jacs.0c08020. [DOI] [PubMed] [Google Scholar]
- a Bierschenk S. M.; Pan J. Y.; Settineri N. S.; Warzok U.; Bergman R. G.; Raymond K. N.; Toste F. D. Impact of host flexibility on selectivity in a supramolecular host-catalyzed enantioselective aza-Darzens reaction. J. Am. Chem. Soc. 2022, 144, 11425–11433. 10.1021/jacs.2c04182. [DOI] [PubMed] [Google Scholar]; b Xue W.; Ronson T. K.; Lu Z.; Nitschke J. R. Solvent drives switching between Λ and Δ metal center stereochemistry of M8L6 cubic cages. J. Am. Chem. Soc. 2022, 144, 6136–6142. 10.1021/jacs.2c00245. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Li S.-C.; Cai L.-X.; Hong M.; Chen Q.; Sun Q.-F. Combinatorial self-assembly of coordination cages with systematically fine-tuned cavities for efficient co-encapsulation and catalysis. Angew. Chem., Int. Ed. 2022, 61, e2022047. 10.1002/anie.202204732. [DOI] [PubMed] [Google Scholar]
- a McTernan C. T.; Davies J. A.; Nitschke J. R. Beyond platonic: how to build metal–organic polyhedra capable of binding low-symmetry, information-rich molecular cargoes. Chem. Rev. 2022, 122, 10393–10437. 10.1021/acs.chemrev.1c00763. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Crowley J. D.; Lisboa L. S.; van Hilst Q. V. C.. Supramolecular systems: metallo-molecular machines and stimuli responsive metallo-macrocycles and cages. In Comprehensive Coordination Chemistry III; Constable E. C., Parkin G., Que L. Jr., Eds.; Elsevier: Oxford, 2021; pp 174–205. [Google Scholar]; c Cai L.-X.; Yan D.-N.; Cheng P.-M.; Xuan J.-J.; Li S.-C.; Zhou L.-P.; Tian C.-B.; Sun Q.-F. Controlled self-assembly and multistimuli-responsive interconversions of three conjoined twin-cages. J. Am. Chem. Soc. 2021, 143, 2016–2024. 10.1021/jacs.0c12064. [DOI] [PubMed] [Google Scholar]; d Ghosh A.; Schmittel M. Using multiple self-sorting for switching functions in discrete multicomponent systems. Beilstein J. Org. Chem. 2020, 16, 2831–2853. 10.3762/bjoc.16.233. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Xue M.; Yang Y.; Chi X.; Yan X.; Huang F. Development of pseudorotaxanes and rotaxanes: from synthesis to stimuli-responsive motions to applications. Chem. Rev. 2015, 115, 7398–7501. 10.1021/cr5005869. [DOI] [PubMed] [Google Scholar]
- a Kim T. Y.; Vasdev R. A. S.; Preston D.; Crowley J. D. Strategies for reversible guest uptake and release from metallosupramolecular architectures. Chem.—Eur. J. 2018, 24, 14878–14890. 10.1002/chem.201802081. [DOI] [PubMed] [Google Scholar]; b Zhiquan L.; Xie H.; Border S. E.; Gallucci J.; Pavlovic R. Z.; Badjic J. D. A Stimuli-responsive molecular capsule with switchable dynamics, chirality, and encapsulation characteristics. J. Am. Chem. Soc. 2018, 140, 11091–11100. 10.1021/jacs.8b06190. [DOI] [PubMed] [Google Scholar]; c Preston D.; Fox-Charles A.; Lo W. K.; Crowley J. D. Chloride triggered reversible switching from a metallosupramolecular [Pd2L4]4+ cage to a [Pd2L2Cl4] metallo-macrocycle with release of endo- and exo-hedrally bound guests. Chem. Commun. 2015, 51, 9042–9045. 10.1039/C5CC02226F. [DOI] [PubMed] [Google Scholar]
- a Ding C.; Tong L.; Feng J.; Fu J. Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules 2016, 21, 1715. 10.3390/molecules21121715. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Cullen W.; Turega S.; Hunter C. A.; Ward M. D. pH-dependent binding of guests in the cavity of a polyhedral coordination cage: reversible uptake and release of drug molecules. Chem. Sci. 2015, 6, 625–631. 10.1039/C4SC02090A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ronson T. K.; Carpenter J. P.; Nitschke J. R. Dynamic optimization of guest binding in a library of diastereomeric heteroleptic coordination cages. Chem 2022, 8, 557–568. 10.1016/j.chempr.2021.12.017. [DOI] [Google Scholar]; b Roy A.; Singh S.; Bajpai J.; Bajpai A. Controlled pesticide release from biodegradable polymers. Cent. Eur. J. Chem. 2014, 12, 453–469. 10.2478/s11532-013-0405-2. [DOI] [Google Scholar]
- a Zhang D.; Ronson T. K.; Zou Y.-Q.; Nitschke J. R. Metal–organic cages for molecular separations. Nat. Rev. Chem. 2021, 5, 168–182. 10.1038/s41570-020-00246-1. [DOI] [PubMed] [Google Scholar]; b Rajbanshi A.; Moyer B. A.; Custelcean R. Sulfate separation from aqueous alkaline solutions by selective crystallization of alkali metal coordination capsules. Cryst. Growth Des. 2011, 11, 2702–2706. 10.1021/cg200515w. [DOI] [Google Scholar]
- a Fuertes-Espinosa C.; Murillo J.; Soto M. E.; Ceron M. R.; Morales-Martínez R.; Rodríguez-Fortea A.; Poblet J. M.; Echegoyen L.; Ribas X. Highly selective encapsulation and purification of U-based C78-EMFs within a supramolecular nanocapsule. Nanoscale 2019, 11, 23035–23041. 10.1039/C9NR07660C. [DOI] [PubMed] [Google Scholar]; b García-Simón C.; Garcia-Borràs M.; Gómez L.; Parella T.; Osuna S.; Juanhuix J.; Imaz I.; Maspoch D.; Costas M.; Ribas X. Sponge-like molecular cage for purification of fullerenes. Nat. Commun. 2014, 5, 5557. 10.1038/ncomms6557. [DOI] [PubMed] [Google Scholar]
- pH as a stimulus to trigger transformation processes that lead to structural conversions:; a Kishimoto M.; Kondo K.; Akita M.; Yoshizawa M. A pH-responsive molecular capsule with an acridine shell: catch and release of large hydrophobic compounds. Chem. Commun. 2017, 53, 1425–1428. 10.1039/C6CC09094J. [DOI] [PubMed] [Google Scholar]; b Kim S. H.; Kim K. R.; Ahn D. R.; Lee J. E.; Yang E. G.; Kim S. Y. Reversible regulation of enzyme activity by pH-responsive encapsulation in DNA nanocages. ACS Nano 2017, 11, 9352–9359. 10.1021/acsnano.7b04766. [DOI] [PubMed] [Google Scholar]; Temperature as a stimulus to trigger transformation processes that lead to structural conversions:; c Wang S.; Yao C.; Ni M.; Xu Z.; Cheng M.; Hu X.-Y.; Shen Y.-Z.; Lin C.; Wang L.; Jia D. Thermo- and oxidation-responsive supramolecular vesicles constructed from self-assembled pillar[6]arene-ferrocene based amphiphilic supramolecular diblock copolymers. Polym. Chem. 2017, 8, 682–688. 10.1039/C6PY01961G. [DOI] [Google Scholar]; Solvent as a stimulus to trigger transformation processes that lead to structural conversions:; d Kilbas B.; Mirtschin S.; Scopelliti R.; Severin K. A solvent-responsive coordination cage. Chem. Sci. 2012, 3, 701–704. 10.1039/C1SC00779C. [DOI] [Google Scholar]; Concentration as a stimulus to trigger transformation processes that lead to structural conversions:; e Lu X.; Li X.; Guo K.; Xie T. Z.; Moorefield C. N.; Wesdemiotis C.; Newkome G. R. Probing a hidden world of molecular self-assembly: concentration-dependent, three-dimensional supramolecular interconversions. J. Am. Chem. Soc. 2014, 136, 18149–18155. 10.1021/ja511341z. [DOI] [PubMed] [Google Scholar]; Other chemical signals as stimuli to trigger transformation processes that lead to structural conversions:; f Zhang D.; Ronson T. K.; Xu L.; Nitschke J. R. Transformation network culminating in a heteroleptic Cd6L6L′2 twisted trigonal prism. J. Am. Chem. Soc. 2020, 142, 9152–9157. 10.1021/jacs.0c03798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wezenberg S. J. Photoswitchable molecular tweezers: isomerization to control substrate binding, and what about vice versa?. Chem. Commun. 2022, 58, 11045–11058. 10.1039/D2CC04329G. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Oldknow S.; Martir D. R.; Pritchard V. E.; Blitz M. A.; Fishwick C. W. G.; Zysman-Colman E.; Hardie M. J. Structure-switching M3L2 Ir(III) coordination cages with photo-isomerising azo-aromatic linkers. Chem. Sci. 2018, 9, 8150–8159. 10.1039/C8SC03499K. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Diaz-Moscoso A.; Ballester P. Light-responsive molecular containers. Chem. Commun. 2017, 53, 4635–4652. 10.1039/C7CC01568B. [DOI] [PubMed] [Google Scholar]
- a Lee S.; Flood A. H. Photoresponsive receptors for binding and releasing anions. J. Phys. Org. Chem. 2013, 26, 79–86. 10.1002/poc.2973. [DOI] [Google Scholar]; b Kishi N.; Akita M.; Kamiya M.; Hayashi S.; Hsu H. F.; Yoshizawa M. Facile catch and release of fullerenes using a photoresponsive molecular tube. J. Am. Chem. Soc. 2013, 135, 12976–12979. 10.1021/ja406893y. [DOI] [PubMed] [Google Scholar]; c Wang H.; Liu F.; Helgeson R. C.; Houk K. N. Reversible photochemically gated transformation of a hemicarcerand to a carcerand. Angew. Chem., Int. Ed. 2013, 52, 655–659. 10.1002/anie.201205376. [DOI] [PubMed] [Google Scholar]
- Han M.; Michel R.; He B.; Chen Y. S.; Stalke D.; John M.; Clever G. H. Light-triggered guest uptake and release by a photochromic coordination cage. Angew. Chem., Int. Ed. 2013, 52, 1319–1323. 10.1002/anie.201207373. [DOI] [PubMed] [Google Scholar]
- Lee H.; Tessarolo J.; Langbehn D.; Baksi A.; Herges R.; Clever G. H. Light-Powered Dissipative Assembly of Diazocine Coordination Cages. J. Am. Chem. Soc. 2022, 144, 3099–3105. 10.1021/jacs.1c12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase T.; Sato S.; Fujita M. Switching the interior hydrophobicity of a self-assembled spherical complex through the photoisomerization of confined azobenzene chromophores. Angew. Chem., Int. Ed. 2007, 119, 5225–5228. 10.1002/ange.200700793. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Ronson T. K.; Nitschke J. R. Functional capsules via subcomponent self-assembly. Acc. Chem. Res. 2018, 51, 2423–2436. 10.1021/acs.accounts.8b00303. [DOI] [PubMed] [Google Scholar]
- Xu L.; Zhang D.; Ronson T. K.; Nitschke J. R. Improved acid resistance of a metal–organic cage enables cargo release and exchange between hosts. Angew. Chem., Int. Ed. 2020, 59, 7435–7438. 10.1002/anie.202001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A.; Bilbeisi R. A.; Ronson T. K.; Zarra S.; Woodhead C.; Nitschke J. R. Selective encapsulation and sequential release of guests within a self-sorting mixture of three tetrahedral cages. Angew. Chem., Int. Ed. 2014, 53, 4556–4560. 10.1002/anie.201400541. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Ronson T. K.; Güryel S.; Thoburn J. D.; Wales D. J.; Nitschke J. R. Temperature controls guest uptake and release from Zn4L4 tetrahedra. J. Am. Chem. Soc. 2019, 141, 14534–14538. 10.1021/jacs.9b07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardi R. G.; Douglas A. O.; Tian R.; Price J. R.; Tajik M.; Donald W. A.; Beves J. E. Visible-light-responsive self-assembled complexes: improved photoswitching properties by metal ion coordination. Angew. Chem., Int. Ed. 2022, e202205701. 10.1002/anie.202205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoshi T.; Takashima S.; Yamagishi T.-a. Photocontrolled Reversible Guest Uptake, Storage, and Release by Azobenzene-Modified Microporous Multilayer Films of Pillar[5]arenes. J. Am. Chem. Soc. 2018, 140, 1544–1548. 10.1021/jacs.7b12893. [DOI] [PubMed] [Google Scholar]
- Jang D.; Pramanik S. K.; Das A.; Baek W.; Heo J.-M.; Ro H.-J.; Jun S.; Park B. J.; Kim J.-M. Photoinduced Reversible Bending and Guest Molecule Release of Azobenzene-Containing Polydiacetylene Nanotubes. Sci. Rep 2019, 9, 15982. 10.1038/s41598-019-52462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L. L. K.; Riddell I. A.; Smulders M. M. J. Self-Assembly of Functional Discrete Three-Dimensional Architectures in Water. Angew. Chem., Int. Ed. 2019, 58, 1280–1307. 10.1002/anie.201806297. [DOI] [PubMed] [Google Scholar]
- Vapaavuori J.; Goulet-Hanssens A.; Heikkinen I. T. S.; Barrett C. J.; Priimagi A. Are two azo groups better than one? investigating the photoresponse of polymer-bisazobenzene complexes. Chem. Mater. 2014, 26, 5089–5096. 10.1021/cm5023129. [DOI] [Google Scholar]
- Ferguson A.; Staniland R. W.; Fitchett C. M.; Squire M. A.; Williamson B. E.; Kruger P. E. Variation of guest selectivity within [Fe4L4]8+ tetrahedral cages through subtle modification of the face-capping ligand. Dalton Trans. 2014, 43, 14550–14553. 10.1039/C4DT02337D. [DOI] [PubMed] [Google Scholar]
- Mosquera J.; Szyszko B.; Ho S. K. Y; Nitschke J. R. Sequence-selective encapsulation and protection of long peptides by a self-assembled FeII8L6 cubic cage. Nat. Commun. 2017, 8, 14882. 10.1038/ncomms14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ghosh A.; Paul I.; Schmittel M. Time-dependent pulses of lithium ions in cascaded signaling and out-of-equilibrium (supra)molecular logic. J. Am. Chem. Soc. 2019, 141, 18954–18957. 10.1021/jacs.9b10763. [DOI] [PubMed] [Google Scholar]; b Pramanik S.; Aprahamian I. Hydrazone switch-based negative feedback loop. J. Am. Chem. Soc. 2016, 138, 15142–15145. 10.1021/jacs.6b10542. [DOI] [PubMed] [Google Scholar]; c Raymo F. M.; Giordani S. Signal communication between molecular switches. Org. Lett. 2001, 3, 3475–3478. 10.1021/ol016502e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


