Figure 1. PGC-1α complexes with the CBC, Mediator, p-TEFb, ERRα and RNAPII during PPP.
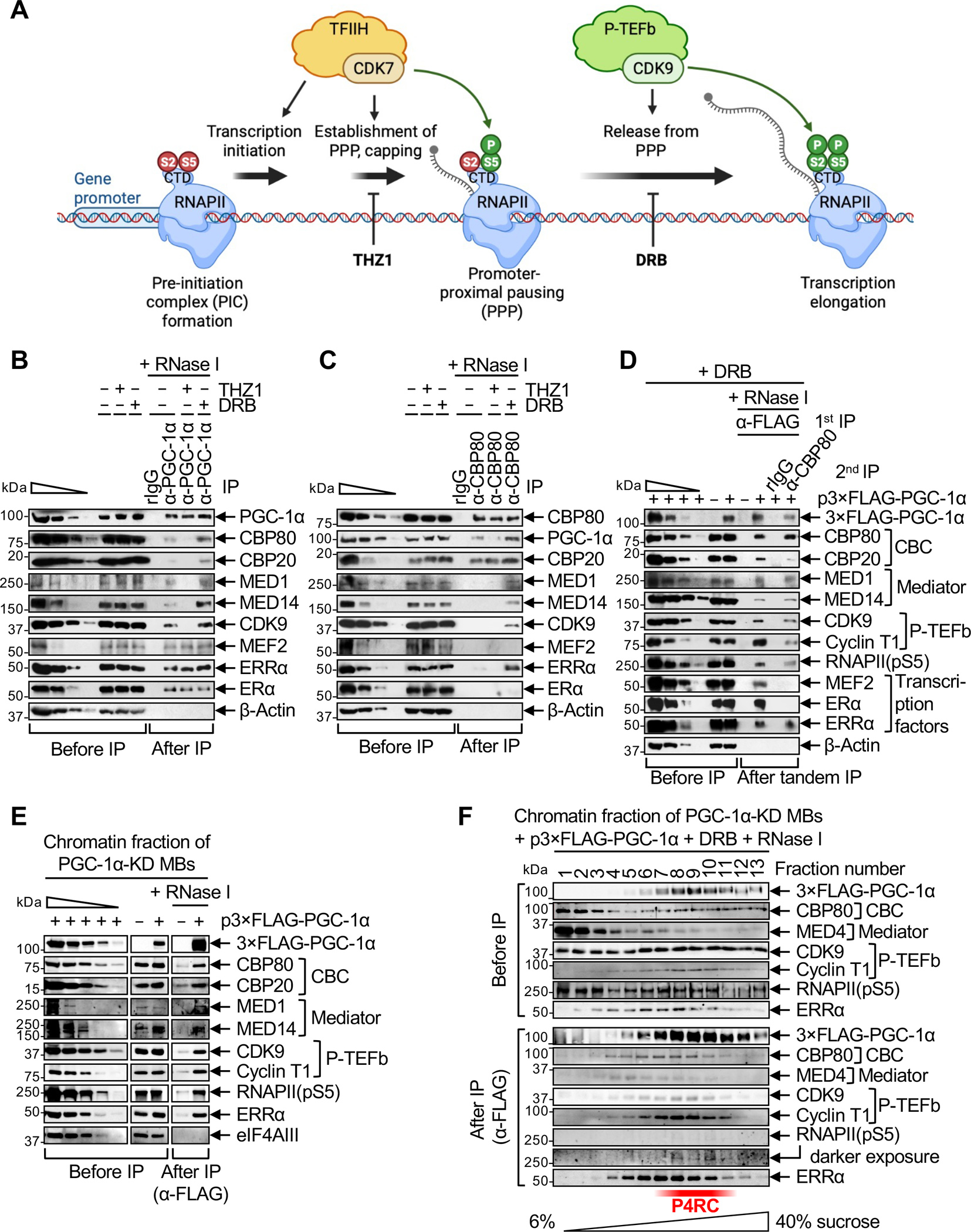
(A) Schematic illustrating the effect of culturing cells in the presence of the CDK inhibitors THZ1 or DRB on early steps of gene transcription, i.e. establishment of promoter-proximal pausing (PPP) and release of RNAPII from PPP into transcription elongation, respectively.
(B) Western blots (WBs) of lysates of C2C12-MBs that were (+) or were not (−) treated with THZ1 or DRB, before or after IP in the presence of RNase I using anti(α)-PGC-1α or, as a control, rabbit (r)IgG. In this WB and others, β-Actin serves to control for variations in loading and IP specificity, the wedge denotes 3-fold serial dilutions of samples to provide semi-quantitative comparisons, and n = 2–3 biological replicates. For this and all other IPs, cell equivalents in IP lanes relative to before IP lanes are described in Table S1.
(C) As in B, but using anti-CBP80 in place of anti-PGC-1α.
(D) WBs of lysates of PGC-1α-KD MBs transiently transfected with a plasmid (p) producing 3×FLAG-PGC-1α(WT) (+) or FLAG alone (−) and subsequently treated with DRB, before IP, after a first IP in the presence of RNase I using anti-FLAG, or after a second IP on the eluates of the first IP in the presence of RNase I using anti-CBP80 or, as a control, rIgG. Samples were loaded so that the amounts of CBP80 in the first and second IPs are equivalent.
(E) WBs of the solubilized chromatin fraction of PGC-1α-KD MBs transiently expressing 3×FLAG-PGC-1α(WT) or FLAG alone (−), before or after IP in the presence of RNase I using anti-FLAG. eIF4AIII serves to control for variations in loading and to control for IP specificity.
(F) WBs of the solubilized chromatin fraction of PGC-1α-KD MBs transiently expressing 3×FLAG-PGC-1α(WT) and treated with DRB, after fractionation in 6–40% sucrose. Fractions were analyzed before or after anti-FLAG IP in the presence of RNase I.
