Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is strongly associated with cardiovascular disease in the general population. Both conditions seem more frequent in patients with inflammatory bowel disease (IBD). We aimed to assess the effect of NAFLD and liver fibrosis on intermediate–high cardiovascular risk in IBD.
Methods
We prospectively included IBD patients undergoing a routine screening program for NAFLD by transient elastography (TE) with associated controlled attenuation parameter (CAP). NAFLD and significant liver fibrosis were defined as CAP ≥275 dB m−1 and liver stiffness measurement by TE ≥8 kPa, respectively. Cardiovascular risk was assessed with the atherosclerotic cardiovascular disease (ASCVD) risk estimator and categorized as low if <5%, borderline if 5%–7.4%, intermediate if 7.5%–19.9%, and high if ≥20% or if previous cardiovascular event. Predictors of intermediate–high cardiovascular risk were investigated by multivariable logistic regression analysis.
Results
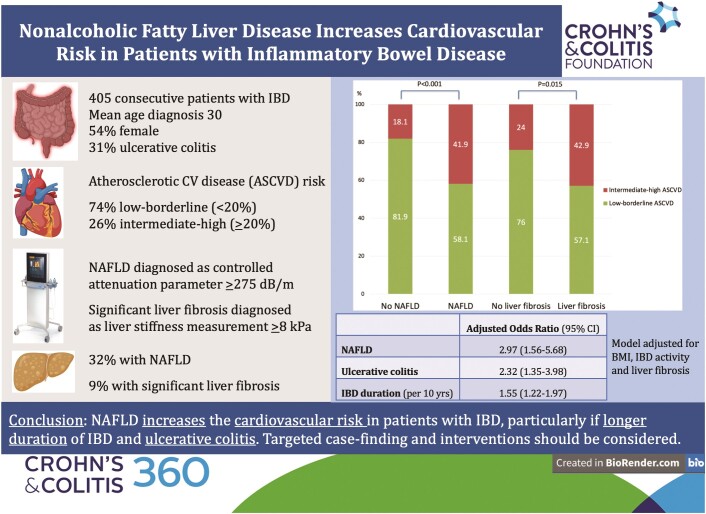
Of 405 patients with IBD included, 278 (68.6%), 23 (5.7%), 47 (11.6%), and 57 (14.1%) were categorized as at low, borderline, intermediate, and high ASCVD risk, respectively. NAFLD and significant liver fibrosis were found in 129 (31.9%) and 35 (8.6%) patients, respectively. After adjusting for disease activity, significant liver fibrosis and body mass index, predictors of intermediate–high ASCVD risk were NAFLD (adjusted odds ratio [aOR] 2.97, 95% CI, 1.56–5.68), IBD duration (aOR 1.55 per 10 years, 95% CI, 1.22–1.97), and ulcerative colitis (aOR 2.32, 95% CI, 1.35–3.98).
Conclusions
Assessment of cardiovascular risk should be targeted in IBD patients with NAFLD, particularly if they have longer IBD duration and ulcerative colitis.
Keywords: atherosclerotic cardiovascular disease risk, controlled attenuation parameter, transient elastography, IBD duration, ulcerative colitis
Graphical Abstract
Graphical Abstract.
Key Messages.
Patients with IBD may have increased prevalence of both NAFLD and cardiovascular disease.
In 405 IBD patients, we investigated the association between NAFLD, diagnosed with controlled attenuation parameter by transient elastography, and the cardiovascular risk assessed with the atherosclerotic cardiovascular disease (ASCVD) risk estimator.
IBD patients with NAFLD had higher proportion of intermediate–high ASCVD risk (41.9%) compared with those without NAFLD (18.1%).
Despite their young age, IBD patients with NAFLD have increased cardiovascular risk, particularly in case of longer IBD duration and of ulcerative colitis.
Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a spectrum of liver pathologies ranging from benign hepatic steatosis to nonalcoholic steatohepatitis (NASH).1 NAFLD is the most prevalent liver disease in North America and predicted to become the leading cause for liver transplantation in coming years.2 This increasing burden is driven by the upward trajectories of obesity and type 2 diabetes at a global level. Indeed, classical risk factors for NAFLD include the metabolic conditions encompassed into the definition of metabolic syndrome, such as insulin resistance, obesity, and dyslipidemia.1 NAFLD seems more frequent in patients with inflammatory bowel diseases (IBD). A recent meta-analysis found an overall pooled prevalence at 27.5%, with associated predictors being older age, metabolic risk factors, methotrexate use, prior surgery, and longer duration of IBD.3 This increased prevalence may be due to a more complex pathogenesis linking IBD with NAFLD, including chronic inflammation, gut dysbiosis, and long-term use of medications.4
NAFLD and its comorbid conditions extend beyond the liver. It is a multisystem clinical disease based on a chronic inflammatory milieu resulting in a wide range of extrahepatic manifestations, notably cardiovascular disease (CVD) which is the leading cause of mortality in this patient population.5,6 Classical CVD risk factors, such as hypertension, dyslipidemia, insulin resistance, and central obesity, are encapsulated by the metabolic syndrome but there is growing evidence that NAFLD may confer an additional risk of CVD. NAFLD is associated with higher risk of cardiovascular events and mortality independent of features of metabolic syndrome.7 The correlation between NAFLD and CVD may be even more peculiar in people with IBD because of IBD-specific factors interfering in this complex interplay. Several studies showed increased risk of cardiovascular complications, especially during IBD relapses.8 However, individuals with IBD present with lower prevalence of classic CVD risk factors, like high body mass index (BMI) or lipid disturbances, compared to the general population. This paradox, also observed in other chronic inflammatory diseases, is linked with the negative impact of global inflammation on endothelium and thrombophilia. Endothelium plays a role in physiologic regulation of vascular tone, cell adhesion, migration, and resistance to thrombosis and its dysfunction is associated with increased risk of atherosclerosis development.8
We conducted a prospective cross-sectional study to evaluate the association between NAFLD with intermediate–high cardiovascular risk in patients with IBD by employing transient elastography (TE) with controlled attenuation parameter (CAP) and serum biomarkers.
Patients and Methods
Study Design and Population
We performed a cohort study at the McGill University Health Centre (MUHC) IBD Centre, which follows about 5000 active adult patients. Consecutive adult patients with IBD were approached and invited to participate in the study by undergoing TE with CAP as part of a screening program for liver disease, between October 2015 and December 2020. Exclusion criteria were the following: (1) positivity for hepatitis C virus (HCV) antibody or hepatitis B virus (HBV) surface antigen; (2) history of preexisting liver disease or new diagnosis at the baseline screening testing (auto-immune hepatitis, primary biliary cholangitis, primary sclerosing cholangitis, hemochromatosis, Wilson’s disease, and alpha-1 anti-trypsin); (3) history of hepatocellular carcinoma, liver transplantation, or decompensated liver disease (ascites, hepatorenal syndrome, spontaneous bacterial peritonitis, hepatic encephalopathy, and variceal hemorrhage); (4) hazardous alcohol intake, as estimated by an Alcohol Use Disorders Identification Test (AUDIT-C) score ≥79; (5) pregnancy at time of recruitment; (6) failure or unreliable measurement of TE. The manuscript was prepared according to the STROBE Statement checklist of items. All patients provided written informed consent for participation into the study. The Research Ethic Board of the Research Institute of the MUHC approved the study (study code 14-026-GEN 2015-1134), which was conducted according to the Declaration of Helsinki.
Outcome Measures
The primary study outcome was to determine predictors of cardiovascular risk, assessed using the atherosclerotic cardiovascular disease (ASCVD) risk score estimator proposed by the 2018 American College of Cardiology (ACC) and the American Heart Association (AHA) guidelines.10 This tool includes age, sex, race, lipid pattern, blood pressure, hypertension, and diabetes treatment and smoking as risk factors. According to ACC/AHA guidelines, the 10-year risk for CVD was computed in patients aged between 40 and 75 years and categorized as low (<5%), borderline (5%–7.4%), intermediate (7.5%–19.9%), and high (≥20%). Patients with previous clinical cardiovascular events were considered at high risk. Clinical cardiovascular events were defined as prior myocardial infarction, angina, stroke, or CVD equivalent, such as peripheral arterial disease.
Noninvasive Diagnostic Tests for NAFLD and Liver Fibrosis
Liver stiffness measurement (LSM) by TE examination was performed on a 3-hour fasting patient by 1 experienced operator (>500 examinations prior to this study). The standard M probe was used initially in all patients. The XL probe was performed in case of failure with M probe and if BMI was ≥30 kg m−2. At least 10 validated measures and an interquartile range (IQR) <30% of the median were required for the examination to be considered valid.11,12 Any grade NAFLD (>5% of hepatocytes) was defined as CAP ≥275 decibels per meter (dB m−1), as per recent guidelines of the European Association for the Study of the Liver.13 Significant liver fibrosis (stage ≥F2 out of 4) was defined as LSM ≥8 kPa. We also calculated a noninvasive marker of NASH, the Fibroscan-aspartate aminotransferase (AST) score (FAST). As previously described, an FAST >0.35 has 90% sensitivity and 50% specificity to diagnose NASH with elevated NAFLD Activity Score (≥4) and significant liver fibrosis (≥F2).14 Finally, the simple biomarkers fibrosis-4 score (FIB-4) and hepatic steatosis index (HSI) were also computed, as previously described.15–17
Clinical, Endoscopic, Radiologic, and Biological Parameters
A complete evaluation of anthropometric, clinical, and biochemical data was performed at enrollment. Biological parameters, collected at time of recruitment and within 3 months from TE examination, included: C-reactive protein (CRP), fecal calprotectin, platelets, bilirubin, AST, alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), albumin, total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides, and glycosylated hemoglobin (HbA1c). In addition, all patients were screened for preexisting liver disease with a panel including: HBV and HCV serologies, anti-nuclear antibody, anti-mitochondrial antibody, anti-smooth muscle antibody, ferritin, ceruloplasmin, and alpha-1 anti-trypsin. The partial Mayo score for ulcerative colitis and the Harvey–Bradshaw Index for Crohn’s disease were used to stratify patients according to their disease activity. Active IBD was defined as partial Mayo score ≥2 for ulcerative colitis and Harvey–Bradshaw Index >4 for Crohn’s disease. Disease phenotype at diagnosis was classified according to the Montreal classification system.18 The diagnosis of diabetes was based on treatment with antidiabetic drugs or the International Diabetes Federation definition.19 The standard questionnaire AUDIT-C was employed to define any alcohol intake (AUDIT-C score ≥5) in each patient. Abdominal ultrasound was performed by 3 experienced radiologists at the Department of Radiology of the MUHC. Hepatic steatosis was diagnosed by the radiologist on the basis of characteristic imaging findings, namely, bright liver pattern, liver–kidney contrast, vascular blurring, and deep hepatic attenuation.20
Statistical Analysis
We based our sample size calculation on the prevalence of intermediate–high ASCVD category in previous IBD studies.21 We assumed the prevalence of intermediate–high ASCVD category would be 18% and 31% in patients with and without NAFLD, respectively (odds ratio 2.1). Given a desired precision of 5%, we calculated 370 patients would be required, with 95% CI. We increased our target sample by 10% in order to account for contingencies, such as failure of TE examination. Analyses were conducted comparing groups at lower (low and borderline ASCVD risk categories) and higher (intermediate and high ASCVD risk categories) cardiovascular risk. Univariate analysis was performed using Student’s t-test for continuous variables and the chi-squared test for categorical variables. A sensitivity analysis was used to investigate the association between NAFLD and ASCVD also in younger IBD patients, based on an age cutoff of 55 years. Predictors of intermediate–high ASCVD were determined using adjusted logistic regression analysis. Covariates were selected and included a priori based on clinical relevance. Covariates used to calculate the ASCVD risk score were excluded. Given the biologic interrelation between NAFLD and liver fibrosis, an interaction term was included in the logistic regression models to consider the investigated relation with the outcome no longer multiplicative. To establish which of the models had the best goodness-of-fit measure, the corrected Akaike information criteria (AIC) and the Bayesian information criteria (BIC) were calculated and compared. A lower AIC and/or BIC indicated a better fit. We reported results as adjusted odds ratios (aORs) with 95% CI. A complete case analysis was used for the multivariable models and the percentage of missing data was less than 10%, unless specified. Kappa statistics was used to measure agreement between CAP and abdominal ultrasound, available in 125 (31%) of the study population. All tests were 2-tailed, with a significance level of α = 0.05. Statistical analyses were performed using STATA 17.0 (STATA Corp. LP).
Results
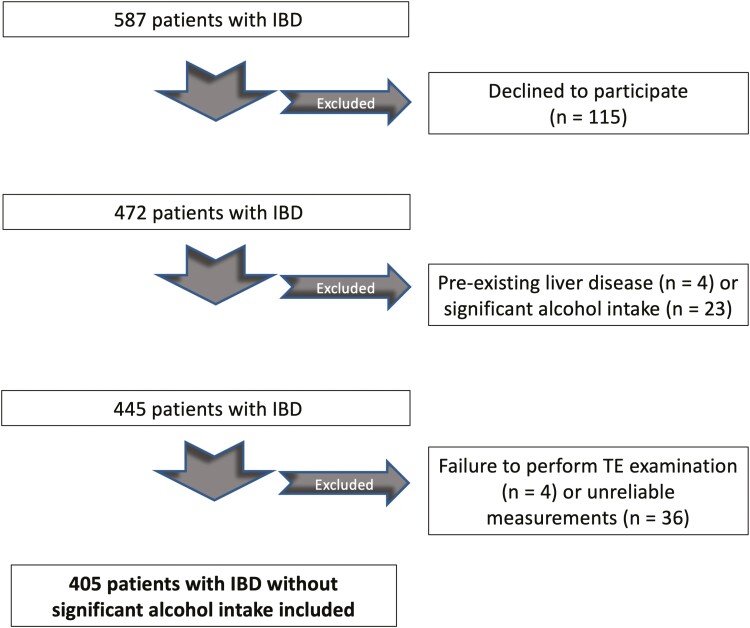
After applying exclusion criteria, 405 patients were included into the study (Figure 1). The failure rate of TE examination (9%) was in line with previous studies.22 The main reason for failure of TE leading to exclusion was unreliable TE examination, including less than 10 validated measures obtained and/or IQR >30%. The XL probe was used in 63 (15.6%) cases, while the standard M probe was applied in the remaining patients. Overall, 219 (54.1%) were female, mean age was 45.1 ± 15.3 years, mean BMI was 25.8 ± 5.0 kg m−2. Diabetes and hypertension affected 29 (7.2%) and 55 (13.6%) patients, respectively. Distribution of IBD type was as follows: 278 (68.6%) had Crohn’s disease and 127 (31.4%) had ulcerative colitis. 115 (28.4%) patients had active IBD and 95 (23.5%) had history of IBD-related surgery. The most common treatment regimens for IBD included anti-tumor necrosis factor therapy (36.0%) and 5-aminosalicylic acid (30.0%). NAFLD and significant liver fibrosis diagnosed by TE with CAP affected 129 (31.9%) and 35 (8.6%) patients, respectively. NASH with liver fibrosis diagnosed by FAST score was found in 11 (2.7%) patients. The agreement between CAP and ultrasound in the subgroup of patients with available abdominal imaging was 0.24 by kappa, corresponding to a fair agreement (proportion of agreement: 64%).
Figure 1.
Flow chart displaying the selection of participants in the study cohort.
ASCVD Risk Categories
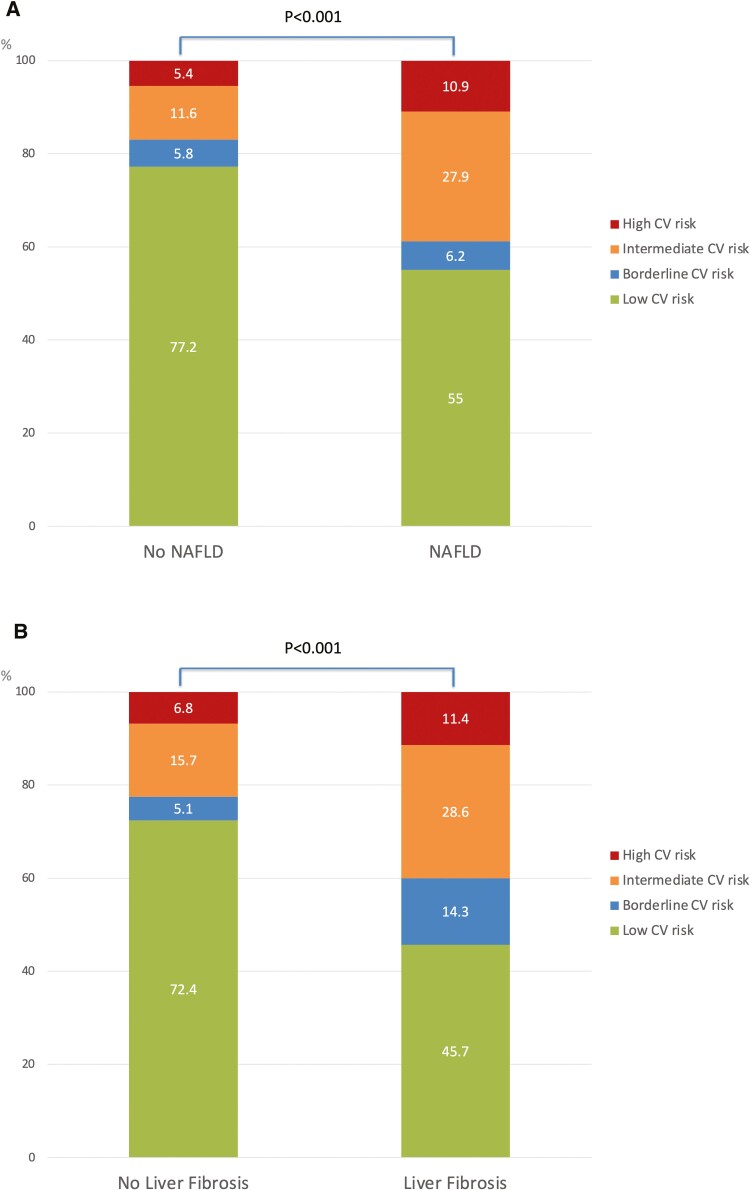
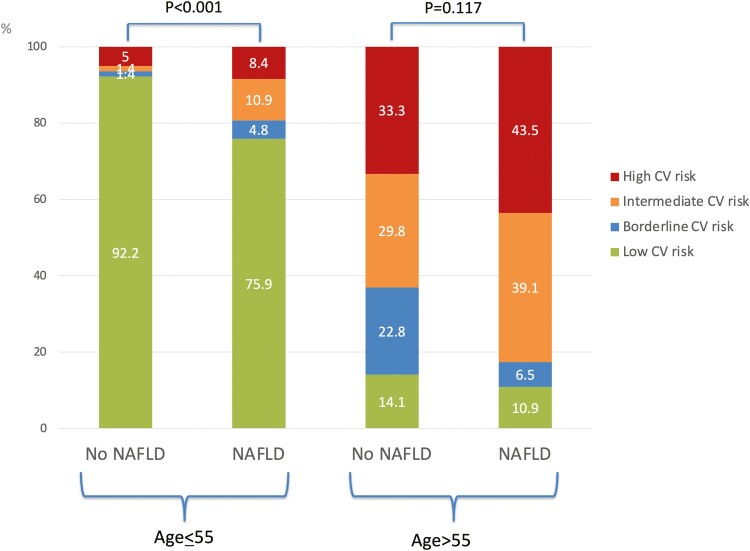
Overall, 278 (68.6%), 23 (5.7%), 47 (11.6%), and 57 (14.1%) patients were categorized as at low, borderline, intermediate, and high ASCVD risk, respectively. Patients with intermediate–high ASCVD risk category were older, more likely to be male and smokers. They also had higher BMI, were older at IBD diagnosis and had longer IBD duration. Patients with intermediate–high ASCVD risk category were more likely to be diabetic, hypertensive and to have Crohn’s disease. They were also more likely to be on 5-aminosalicylic acid, methotrexate, and lipid-lowering drugs and less likely to be on azathioprine/6-mercaptopurine. They had lower platelets and HDL cholesterol and higher GGT, ALP, HbA1c, and triglycerides. Finally, patients with intermediate–high ASCVD risk category had higher noninvasive steatosis tests, including CAP and HSI, and higher liver fibrosis tests, including LSM and FIB-4 (Table 1). Patients with NAFLD and with significant liver fibrosis diagnosed by TE with CAP had higher proportion of intermediate–high ASCVD risk category (Figure 2). ASCVD was increased also in IBD patients <55 years old with NAFLD (Figure 3). There was no significant difference in disease phenotype by the Montreal classification system according to the ASCVD category (Supplementary Figure S1). Patients in the intermediate–high ASCVD risk category had higher proportions of IBD diagnoses over the age of 40, but this was due to the fact that the ASCVD risk score estimator includes age in its formula.
Table 1.
Demographic, clinical, biochemical, histologic, and pharmacotherapeutic characteristics of the study population and univariable analysis by low–borderline vs intermediate–high ASCVD risk score category (n = 405).
| ASCVD risk categories | |||
|---|---|---|---|
| Intermediate–high (n = 104) | Low–borderline (n = 301) | P | |
| Age (years) | 64.1 (9.8) | 39.8 (12.1) | <.001 |
| Male sex (%) | 63 (60.6) | 123 (40.9) | .001 |
| Ethnicity (%) | |||
| White/Caucasian | 88 (84.6) | 249 (82.7) | .451 |
| Black non-Hispanic | 6 (5.8) | 15 (5.0) | |
| Other | 10 (9.6) | 37 (12.3) | |
| Any alcohol intake (%) | 8 (7.7) | 23 (7.6) | .220 |
| Smoking ever (%) | 75 (72.1) | 104 (34.6) | <.001 |
| Diabetes (%) | 27 (26.0) | 2 (0.7) | <.001 |
| Hypertension (%) | 41 (39.4) | 14 (4.7) | <.001 |
| BMI (kg m−2) | 26.7 (4.9) | 25.5 (5.0) | .05 |
| Ulcerative colitis (%) | 42 (40.4) | 85 (28.2) | .021 |
| Age at IBD diagnosis (years) | 43.0 (15.2) | 26.1 (11.4) | <.001 |
| IBD duration (years) | 17.6 (11.5) | 13.3 (10.0) | .001 |
| IBD duration (%) | |||
| <5 years | 15 (17.9) | 69 (82.1) | .038 |
| 5–10 years | 20 (21.1) | 75 (79.0) | |
| >10 years | 69 (30.5) | 157 (69.5) | |
| Active IBD (%) | 25 (24.0) | 91 (30.2) | .228 |
| Prior surgery (%) | 36 (34.6) | 95 (31.6) | .566 |
| History of parenteral nutrition (%) | 10 (9.6) | 33 (11.0) | .700 |
| History of coronary artery disease (%) | 5 (4.8) | 0 | <.001 |
| Medications (%) | |||
| 5-Aminosalicylic acid | 40 (38.5) | 82 (27.2) | .032 |
| Steroids | 9 (8.7) | 36 (12.0) | .355 |
| Azathioprine/6MP | 10 (9.6) | 57 (18.9) | .027 |
| Anti-tumor necrosis factor | 30 (28.8) | 116 (38.5) | .076 |
| Methotrexate | 10 (9.6) | 3 (1.0) | <.001 |
| Lipid-lowering regimen | 29 (27.9) | 12 (4.0) | <.001 |
| Fecal calprotectin (µg g−1) | 402.0 (324.4) | 363.0 (361.1) | .700 |
| Platelet count (109/L) | 243.1 (74.4) | 271.3 (72.9) | .002 |
| AST (IU L−1) | 19.3 (4.3) | 19.7 (7.9) | .729 |
| ALT (IU L−1) | 21.3 (10.2) | 23.1 (20.1) | .417 |
| GGT (IU L−1) | 25.1 (12.1) | 20.6 (15.7) | .033 |
| ALP (IU L−1) | 70.9 (22.2) | 62.9 (20.2) | .003 |
| Albumin (mg L−1) | 4.14 (0.38) | 4.22 (0.39) | .149 |
| HbA1c (%) | 3.3 (2.5) | 1.0 (0.9) | .042 |
| Total cholesterol (mmol L−1) | 4.61 (1.17) | 6.61 (1.09) | .976 |
| LDL cholesterol (mmol L−1) | 2.38 (0.99) | 2.48 (0.95) | .464 |
| HDL cholesterol (mmol L−1) | 1.32 (0.37) | 1.45 (0.39) | .014 |
| Triglycerides (mmol L−1) | 2.03 (1.52) | 1.57 (1.23) | .008 |
| CRP (mg L−1) | 5.0 (4.8) | 5.3 (11.4) | .822 |
| LSM (kPa) | 6.7 (6.6) | 4.9 (2.1) | <.001 |
| CAP (dB m−1) | 673.3 (68.2) | 225.8 (71.4) | <.001 |
| FAST score | 0.09 (0.08) | 0.07 (0.06) | .142 |
| FIB-4 | 1.20 (0.47) | 0.67 (0.32) | <.001 |
| HSI | 36.6 (6.7) | 34.7 (6.9) | .04 |
Continuous variables are expressed as mean ± SD and categorical variables as numbers (%). Any alcohol intake was defined as AUDIT-C ≥5. Active IBD was defined as partial Mayo score ≥2 for ulcerative colitis and Harvey–Bradshaw Index >4 for Crohn’s disease. Normal values for CRP were <5 mg L−1; normal values for fecal calprotectin were <50 µg g−1. The P values refer to t-test or χ2 test between patients with the outcome (intermediate–high ASCVD) and those without the outcome (low–borderline ASCVD). Fecal calprotectin was available in 177 cases. Abbreviations: 6MP, 6-mercaptopurine; ALP, alkaline phosphatase; ALT, alanine aminotransferase; ASCVD, atherosclerotic cardiovascular disease; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled association parameter; CRP, C-reactive protein; FAST, Fibroscan-aspartate aminotransferase; FIB-4, fibrosis-4 score; GGT, gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; HSI, hepatic steatosis index; IBD, inflammatory bowel disease; IU, international unit; LDL, low-density lipoprotein; LSM, liver stiffness measurement; NAFLD, nonalcoholic fatty liver disease.
Figure 2.
Distribution of ASCVD risk categories in the whole study population by (A) NAFLD and (B) significant liver fibrosis status. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease.
Figure 3.
Distribution of ASCVD risk categories in the whole study population by NAFLD status according to age category. Abbreviations: ASCVD, atherosclerotic cardiovascular disease; NAFLD, nonalcoholic fatty liver disease.
Predictors of Intermediate–High ASCVD Risk Category
Table 2 shows the results of multivariable analysis for predictors of intermediate–high ASCVD risk category. After adjusting for BMI, active IBD and significant liver fibrosis, NAFLD (aOR 2.97, 95% CI, 1.56–5.68), longer IBD duration (aOR 1.55 per 10 years, 95% CI, 1.22–1.97), and ulcerative colitis (aOR 2.32, 95% CI, 1.35–3.98) were independently associated with intermediate–high ASCVD risk category. Supplementary Table S1 reports the metabolic and other cardiovascular-sensitive characteristics in patients with ulcerative colitis compared with those with Crohn’s disease. Overall, patients with ulcerative colitis had more cardiovascular-sensitive conditions, including older age and higher total and LDL cholesterol. They were also older at IBD diagnosis and had longer IBD duration and higher fecal calprotectin. Finally, patients with ulcerative colitis were less likely to be smokers and had higher FIB-4 compared with patients with Crohn’s disease.
Table 2.
Multivariable analysis of predictors of intermediate–high cardiovascular risk in patients with IBD.
| Variable | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P |
|---|---|---|---|
| NAFLD (yes vs no) | 3.25 (2.04–5.18) | 2.97 (1.56–5.68) | .001 |
| Liver fibrosis (yes vs no) | 2.37 (1.16–4.82) | 1.06 (0.25–4.55) | .936 |
| BMI (per kg m−2) | 1.04 (0.99–1.09) | 1.00 (0.93–1.06) | .875 |
| Ulcerative colitis (yes vs no) | 1.72 (1.08–2.74) | 2.32 (1.35–3.98) | .002 |
| IBD duration (per 10 years) | 1.45 (1.18–1.79) | 1.55 (1.22–1.97) | <.001 |
| Active IBD (yes vs no) | 0.72 (0.42–1.23) | 0.64 (0.35–1.17) | .145 |
| NAFLD × Fibrosis | — | 1.68 (0.27–10.32) | .576 |
Odds ratios (OR) and 95% CIs are shown for each variable analyzed in univariable and multivariable logistic regression analysis. Abbreviations: BMI, body mass index; IBD, inflammatory bowel disease; NAFLD, nonalcoholic fatty liver disease.
Discussion
This prospective cross-sectional study of consecutive patients with IBD undergoing a routine screening program for liver disease showed that NAFLD diagnosed using TE with CAP predicts increased cardiovascular risk, even after adjusting for IBD-related factors and BMI. A potential deliverable of our finding is the targeted cardiovascular assessment in IBD patients with NAFLD. North America, and especially Canada has among the highest prevalence of IBD worldwide, rendering our findings particularly relevant.23
NAFLD affects one quarter of the general population globally.1 Patients with IBD are potentially at higher risk of NAFLD due to intestinal disease-related conditions which share physiopathologic features with NAFLD, such as chronic relapsing inflammation and immune activation, potentially hepatotoxic drugs, surgery, and parenteral nutrition.4,24 Alteration in gut microbiota has been associated with disease severity in both IBD and NAFLD, thus acting as a potential pathogenic link between the two diseases.4,25 Estimates of NAFLD prevalence among patients with IBD range widely, between 6.2% and 40%.4,16,26 Heterogeneity in study population and design, and in diagnostic tools employed to define NAFLD may account for this discrepancy. In the present prospective study employing a validated diagnostic tool for NAFLD and associated liver fibrosis, that is TE with CAP, we reported a prevalence of NAFLD and significant liver fibrosis at 31.9% and 8.6%, respectively.
NAFLD is a multisystem disease causing several associated conditions. Although liver-related complications are a significant cause of mortality in NAFLD, CVD is the first cause of mortality in these patients, accounting for at least 40% of total deaths. NAFLD is a risk factor for CVD independent of traditional risk factors, such as age, sex, obesity, hypertension, dyslipidemia, diabetes, and tobacco use.27 Because of the strength of this association, guidelines from the American and European Associations for the Study of the Liver recommend cardiovascular risk stratification and prompt use of statins in all patients with NAFLD.28,29 The precise causal mechanisms underlying the association between NAFLD and CVD are incompletely understood, although it is postulated that the drivers of NAFLD progression, including systemic inflammation, dyslipidemia, and endothelial dysfunction, may also underlie accelerated atherogenesis.27 Several studies employed the ASCVD risk score to estimate the cardiovascular risk in NAFLD. A large Korean cohort study found that NAFLD was an independent factor for ≥7.5% estimated cardiovascular risk and that the ASCVD risk score estimator may be appropriate when assessing 10-year CVD risk among individuals with NAFLD.30 Golabi et al demonstrated that, among patients with NAFLD, an ASCVD score ≥7.5% was associated with a higher risk of overall and cardiac-specific mortality.31 To our knowledge, there has been no study investigating the association between cardiovascular risk (as assessed by ASCVD risk score) and NAFLD in patients with IBD. Several studies suggest that IBD may be at increased risk of CVD compared with the general population. A recent study of 66 610 participants (951 with IBD) from the US National Health Interview Survey found an age-adjusted prevalence of ASCVD of 12% in patients with IBD compared with 6.9% on those without IBD.21 A large Canadian study of 35 985 IBD patients from our team found that prevalence of myocardial infarction was higher in IBD patients (3.98%) compared with the Canadian rates (2.0%).32 A meta-analysis of 10 cohort studies found a moderately increased risk of ischemic heart disease in IBD patients (relative risk 1.24, 95% CI, 1.14–1.36).33 Another meta-analysis of 33 observational studies with 72 205 patients with IBD and 891 840 controls revealed an increased risk of thromboembolic events in IBD compared with controls (relative risk 1.96).34,35 The pathogenesis underlying the link between IBD and CVD is not understood. In the general population, the prevalence of traditional ASCVD risk factors such as smoking, diabetes, hypertension, and dyslipidemia correlates with a higher risk of cardiovascular events, but IBD patients may be at higher CVD risk despite lower rates of some of these risk factors.36 Current concepts point at interplay among genetic susceptibility, abnormal mucosal immune response, defective epithelium, and gut microbiome alterations.8 Medications used to treat IBD and target inflammation, such as steroids, may also accelerate CVD by exacerbating ASCVD risk factors.36 These are the same putative mechanisms linking IBD with NAFLD. In IBD, low-grade systemic inflammation linking NAFLD with CVD may be enhanced by the complex interrelationship among diet, uncontrolled intestinal inflammatory process, genetics, dysfunctional visceral adipose tissue, and pro-inflammatory cytokines (interleukin 6, interleukin 1b, and tumor necrosis factor alpha).7 Moreover, the vascular endothelium dysfunction which characterizes both IBD and NAFLD also plays a pivotal role in CVD.8
In our IBD study population, patients with NAFLD had higher prevalence of intermediate–high ASCVD risk score compared with those without NAFLD (41.9% vs 18.1%). This finding was confirmed especially in patients <55 years of age. Similarly, we found that patients with significant liver fibrosis had higher prevalence of intermediate–high ASCVD risk score compared with those without fibrosis (42.9% vs 24.0%). This finding was corroborated by the fact that also serum steatosis and fibrosis biomarkers (HSI and FIB-4) were higher in patients with intermediate–high ASCVD risk score. Conversely, we did not find an increase in FAST score, a NASH biomarker, in patients with intermediate–high ASCVD risk score. This could be due to the fact that the FAST score incorporates AST, which did not seem to be linked to increased ASCVD. On multivariable analysis, NAFLD diagnosed by CAP remained an independent predictor of intermediate–high ASCVD risk score. Significant liver fibrosis, on the contrary, was not independently associated with intermediate–high ASCVD score, likely because the IBD population is a young one, so with less liver fibrosis than other NAFLD populations. Duration of IBD was another independent predictor of intermediate–high ASCVD, likely due to the longer exposure to multiple factors underlying an increased cardiovascular risk in these patients, such as chronic systemic inflammation, abnormal mucosal immune response, and gut dysbiosis. We also found that ulcerative colitis disease was a predictor of intermediate–high ASCVD risk score. Patients with ulcerative colitis had more cardiovascular-sensitive conditions, including older age and higher total and LDL cholesterol, which may explain our findings. Indeed, previous reports described an increased cardiovascular risk in both ulcerative colitis and Crohn’s disease, without specific differentiation between these IBD subtypes.36 These findings suggest that, with advancing age, the traditional risk factors become predictors of CVD even in IBD patients. Along the same lines, a higher proportion of patients in intermediate–high ASCVD risk category were already on statins/lipid lower drugs in our study population.
Our study has several strengths, namely its prospective design with a well-characterized population. Moreover, we employed a validated and accurate diagnostic tool, namely TE with CAP in consecutive IBD patients and paired our findings with serum biomarkers. We wish to acknowledge some limitations of our study. First, the 10-year ASCVD risk score has not been investigated in IBD patient as a predictor of CVD risk. Moreover, this score does not recognize or incorporate the chronic inflammatory process (disease activity or severity) typical of IBD. The Reynolds risk score instead incorporates CRP and it has been previously used in the IBD population, however it has been mostly validated in women and not all required variables were available in our cohort.37,38 Hence, we recognize that ASCVD risk score may not provide a complete assessment of CVD in IBD patients. Second, the cross-sectional study design did not allow us to speculate on the causal association between IBD and CVD. Third, we based the association between NAFLD and CVD on a cardiovascular risk prediction rather than clinical outcomes (cardiovascular events). Fourth, due to the relatively small number of patients exposed, we were not able to tease out the potential contribution of IBD medications to our findings. We found an increased ASCVD score in patients on methotrexate on univariable analysis, while no association with steroids, potentially known to increase ASCVD risk. This may be explained by the fact that steroids were used only short term in our population. Moreover, due to nature of the study design, the recollect data on prior use of steroid was limited, which would have been helpful to assess disease severity in more detail. Fifth, histological diagnosis of NAFLD and fibrosis was not available, as liver biopsy was not feasible even though it remains the gold standard. Sixth, we acknowledged that we did not have a non-IBD control arm for comparison. Finally, our study was conducted at a tertiary care center, potentially limiting generalizability.
In conclusion, in a cohort of IBD patients we showed that NAFLD diagnosed by TE with CAP has a potential value for predicting cardiovascular risk in patients with IBD. Despite their relatively young age, individuals with IBD and NAFLD should be screened for cardiovascular risk with an estimator like ASCVD, especially if the patient has been living with IBD for a longer duration and therefore been exposed to multiple cardio-toxic risk factors. Noninvasive screening strategies could help early diagnosis and initiation of interventions, including cardiovascular risk stratification and initiation of statin. Longitudinal studies aimed at evaluating the impact of early stratification and interventions on cardiovascular morbidity and mortality are warranted.
Supplementary Material
Contributor Information
Dana Kablawi, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
Faisal Aljohani, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
Chiara Saroli Palumbo, Division of Gastroenterology, Jewish General Hospital, Montreal, Quebec, Canada.
Sophie Restellini, Gastroenterology Department, University Hospital of Geneva, Geneva, Switzerland.
Alain Bitton, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
Gary Wild, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
Waqqas Afif, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
Peter L Lakatos, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada; 1st Department of Medicine, Semmelweis University, Budapest, Hungary.
Talat Bessissow, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
Giada Sebastiani, Division of Gastroenterology and Hepatology, McGill University Health Centre, Montreal, Quebec, Canada.
Funding
Echosens (Paris, France) provided the FibroScan used to conduct the present research. G.S. is supported by a Senior Salary Award from Fonds de Recherche du Quebec – Sante (FRQS) (#296306).
Authors’ Contributions
D.K. and F.A. were involved in study concept and design, acquisition of data, and interpretation of data. C.S.P., S.R., G.W., W.A., P.L.L., and A.B. were involved in acquisition of data. T.B. was involved in study concept and design, acquisition and interpretation of data, critical revision of manuscript, and overall study supervision. G.S. was involved in study concept and design, acquisition and interpretation of data, analysis and drafting of manuscript, critical revision of manuscript, and overall study supervision. All the authors declare they have participated in the preparation of the manuscript and have seen and approved the final version.
Conflicts of Interest
A.B. has been a member of Advisory Boards for AbbVie, Pfizer, Takeda, Janssen, and Merck, and speaker’s bureaus for AbbVie, Janssen, Takeda, and Pfizer. W.A. has been a speaker for Janssen, Prometheus, Dynacare, Takeda, and AbbVie Theradiag. P.L.L. has been a speaker and/or advisory board member for AbbVie, Arena, Falk Pharma GmbH, Ferring, Genetech, Janssen, Kyowa Hakko Kirin Pharma, Mitsubishi Tanabe Pharma Corporation, MSD, Pfizer, Roche, Shire, Takeda, and Tillots, and has received unrestricted research grants from AbbVie, MSD, and Pfizer. T.B. has received honoraria and acted as a consultant for AbbVie, Alimentiv (formerly Robarts Inc.), Amgen, Bristol-Myers-Squibb, Ferring, Gilead, Janssen, Merck, Pentax, Pfizer, Roche, Sandoz, Takeda, and Viatris. G.S. has acted as speaker for Merck, BMS, Gilead, and AbbVie, served as an advisory board member for Merck and BMS, and has received research funding from Theratechnologies Inc. F.A., D.K., C.S.P., S.R., and G.W. have no conflicts of interest to declare.
Data Availability
According to stipulations of the patient consent form signed by all study participants, ethical restrictions imposed by our Institutional Ethics review boards (Institutional Ethics Review Board Biomedical B Research Ethics Board of the McGill University Health Centre), and legal restrictions imposed by Canadian law regarding clinical trials, anonymized data are available upon reasonable request. Please send data access requests to Sheldon Levy, Biomedical B (BMB) Research Ethics Board (REB) Coordinator Centre for Applied Ethics, 5100, boul. de Maisonneuve Ouest, 5th Floor, Office 576, Montréal, Québec H4A 3T2, Canada.
References
- 1. Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. . AASLD practice guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology. 2023. doi: 10.1097/HEP.0000000000000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA.. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. [DOI] [PubMed] [Google Scholar]
- 3. Zou ZY, Shen B, Fan JG.. Systematic review with meta-analysis: epidemiology of nonalcoholic fatty liver disease in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(11):1764–1772. [DOI] [PubMed] [Google Scholar]
- 4. Restellini S, Chazouilleres O, Frossard JL.. Hepatic manifestations of inflammatory bowel diseases. Liver Int. 2017;37(4):475–489. [DOI] [PubMed] [Google Scholar]
- 5. Dulai PS, Singh S, Patel J, et al. . Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. VanWagner LB, Rinella ME.. Extrahepatic manifestations of nonalcoholic fatty liver disease. Curr Hepatol Rep. 2016;15(2):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Targher G, Byrne CD, Tilg H.. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705. [DOI] [PubMed] [Google Scholar]
- 8. Czubkowski P, Osiecki M, Szymanska E, Kierkus J.. The risk of cardiovascular complications in inflammatory bowel disease. Clin Exp Med. 2020;20(4):481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR.. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31(7):1208–1217. [DOI] [PubMed] [Google Scholar]
- 10. Wilson PWF, Polonsky TS, Miedema MD, Khera A, Kosinski AS, Kuvin JT.. Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1144–e1161. [DOI] [PubMed] [Google Scholar]
- 11. Sandrin L, Fourquet B, Hasquenoph JM, et al. . Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29(12):1705–1713. [DOI] [PubMed] [Google Scholar]
- 12. European Association for Study of Liver, Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. [DOI] [PubMed] [Google Scholar]
- 13. European Association for the Study of the Liver, Electronic address eee; Clinical Practice Guideline Panel; Chair; EASL Governing Board representative; Panel members. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J Hepatol. 2021;75(3):659–689. [DOI] [PubMed] [Google Scholar]
- 14. Newsome PN, Sasso M, Deeks JJ, et al. . FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5(4):362–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vallet-Pichard A, Mallet V, Nalpas B, et al. . FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. [DOI] [PubMed] [Google Scholar]
- 16. Bessissow T, Le NH, Rollet K, Afif W, Bitton A, Sebastiani G.. Incidence and predictors of nonalcoholic fatty liver disease by serum biomarkers in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22(8):1937–1944. [DOI] [PubMed] [Google Scholar]
- 17. Lee JH, Kim D, Kim HJ, et al. . Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508. [DOI] [PubMed] [Google Scholar]
- 18. Silverberg MS, Satsangi J, Ahmad T, et al. . Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 19. International Diabetes Federation Guideline Development Group. Global guideline for type 2 diabetes. Diabetes Res Clin Pract. 2014;104(1):1–52. [DOI] [PubMed] [Google Scholar]
- 20. Hamaguchi M, Kojima T, Itoh Y, et al. . The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102(12):2708–2715. [DOI] [PubMed] [Google Scholar]
- 21. Nasir K, Acquah I, Dey AK, et al. . Inflammatory bowel disease and atherosclerotic cardiovascular disease in U.S. adults—a population-level analysis in the national health interview survey. Am J Prev Cardiol. 2022;9:100316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cassinotto C, Boursier J, de Ledinghen V, et al. . Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63(6):1817–1827. [DOI] [PubMed] [Google Scholar]
- 23. Rocchi A, Benchimol EI, Bernstein CN, et al. . Inflammatory bowel disease: a Canadian burden of illness review. Can J Gastroenterol. 2012;26(11):811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sourianarayanane A, Garg G, Smith TH, Butt MI, McCullough AJ, Shen B.. Risk factors of non-alcoholic fatty liver disease in patients with inflammatory bowel disease. J Crohns Colitis. 2013;7(8):e279–e285. [DOI] [PubMed] [Google Scholar]
- 25. Raman M, Ahmed I, Gillevet PM, et al. . Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2013;11(7):868–875.e1–3. [DOI] [PubMed] [Google Scholar]
- 26. Gizard E, Ford AC, Bronowicki JP, Peyrin-Biroulet L.. Systematic review: The epidemiology of the hepatobiliary manifestations in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2014;40(1):3–15. [DOI] [PubMed] [Google Scholar]
- 27. Przybyszewski EM, Targher G, Roden M, Corey KE.. Nonalcoholic fatty liver disease and cardiovascular disease. Clin Liver Dis (Hoboken). 2021;17(1):19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chalasani N, Younossi Z, Lavine JE, et al. . The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 29. European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388–1402. [DOI] [PubMed] [Google Scholar]
- 30. Lee JI, Kim MC, Moon BS, et al. . The relationship between 10-year cardiovascular risk calculated using the pooled cohort equation and the severity of non-alcoholic fatty liver disease. Endocrinol Metab (Seoul). 2016;31(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi ZM.. Mortality risk detected by atherosclerotic cardiovascular disease score in patients with nonalcoholic fatty liver disease. Hepatol Commun. 2019;3(8):1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Golovics PA, Verdon C, Wetwittayakhlang P, et al. . Increased prevalence of myocardial infarction and stable stroke proportions in patients with inflammatory bowel diseases in Quebec in 1996–2015. J Clin Med. 2022;11(3):686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feng W, Chen G, Cai D, Zhao S, Cheng J, Shen H.. Inflammatory bowel disease and risk of ischemic heart disease: an updated meta-analysis of cohort studies. J Am Heart Assoc. 2017;6(8):e005892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen GC, Bernstein CN, Bitton A, et al. . Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146(3):835–848.e6. [DOI] [PubMed] [Google Scholar]
- 35. Kirchgesner J, Beaugerie L, Carrat F, et al. . Increased risk of acute arterial events in young patients and severely active IBD: a nationwide French cohort study. Gut. 2018;67(7):1261–1268. [DOI] [PubMed] [Google Scholar]
- 36. Bigeh A, Sanchez A, Maestas C, Gulati M.. Inflammatory bowel disease and the risk for cardiovascular disease: does all inflammation lead to heart disease? Trends Cardiovasc Med. 2020;30(8):463–469. [DOI] [PubMed] [Google Scholar]
- 37. Ridker PM, Buring JE, Rifai N, Cook NR.. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–619. [DOI] [PubMed] [Google Scholar]
- 38. Sands BE, Taub PR, Armuzzi A, et al. . Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(1):123–132.e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
According to stipulations of the patient consent form signed by all study participants, ethical restrictions imposed by our Institutional Ethics review boards (Institutional Ethics Review Board Biomedical B Research Ethics Board of the McGill University Health Centre), and legal restrictions imposed by Canadian law regarding clinical trials, anonymized data are available upon reasonable request. Please send data access requests to Sheldon Levy, Biomedical B (BMB) Research Ethics Board (REB) Coordinator Centre for Applied Ethics, 5100, boul. de Maisonneuve Ouest, 5th Floor, Office 576, Montréal, Québec H4A 3T2, Canada.