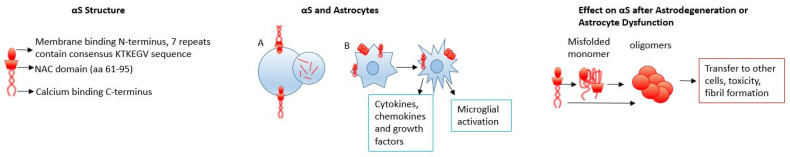
Figure 1.
αS’s structure consists of an amphipathic membrane-binding N-terminus sequence that contains 7 repeats with the consensus KTKEGV sequence, a non-amyloid-β component (NAC) domain responsible for its aggregation potential, and a calcium binding C-terminus. In A, the astrocyte can degrade αS monomers and oligomers through the endolysosomal pathway. In B, interaction with αS monomers and oligomers can cause astrocyte reactivity resulting in the release of cytokines, chemokines and growth factors, and cause microglial activation, although the level of monomeric αS to induce broad effects is uncertain. In the event of astrodegeneration or astrocyte dysfunction, αS can misfold, aggregate and spread from cell to cell, causing toxic fibril formation, which can also then cause native αS to misfold as well.