Figure 22.
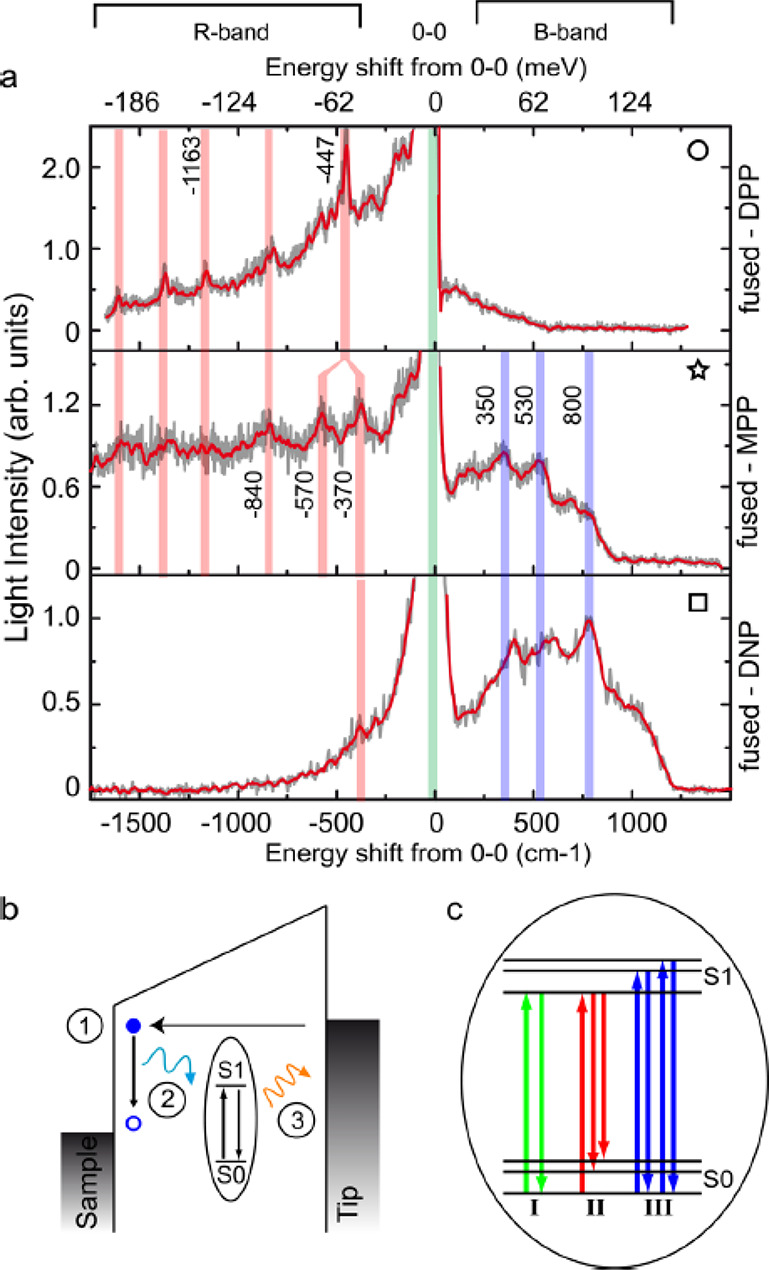
Vibronic features and emission mechanism. (a) Light emission spectra of fused-5,15-(dinaphthalene)-10,20-(dibromo)-porphyrin (DPP), -5-(phenyl)-10,20-(dibromo)-porphyrin (MPP), and -5,15-(dinaphthalene)-10,20-(dibromo)-porphyrin (DNP) plotted as a function of the energy shift from the 0–0 lines. The raw (smoothed) data appear in gray (red). (b) Schematic representation of the emission mechanism. The energy lost by an inelastic tunneling electron (1) is transferred to the molecular emitter that is excited from S0 to S1 (2). Eventually, the emitter relaxes to its electronic ground state by emitting a photon (3). (c) Details of the excitation and de-excitation processes. Three possible paths are described: The green arrows characterize the most efficient path leading to the 0–0 emission line, which corresponds to a molecule excited to the S1 vibrational ground level and then relaxing to the S0 vibrational ground level. The red and blue arrows characterize much less efficient paths. In path II (red) the molecule is also excited to the S1 vibrational ground level but decays to the S0 vibrational excited level (R-band). Path III (blue) corresponds to a molecule excited to the S1 vibrational excited levels which relaxes to the S0 vibrational ground level (B-band). Reproduced with permission from ref (221). Copyright 2016 American Chemical Society.
