Figure 23.
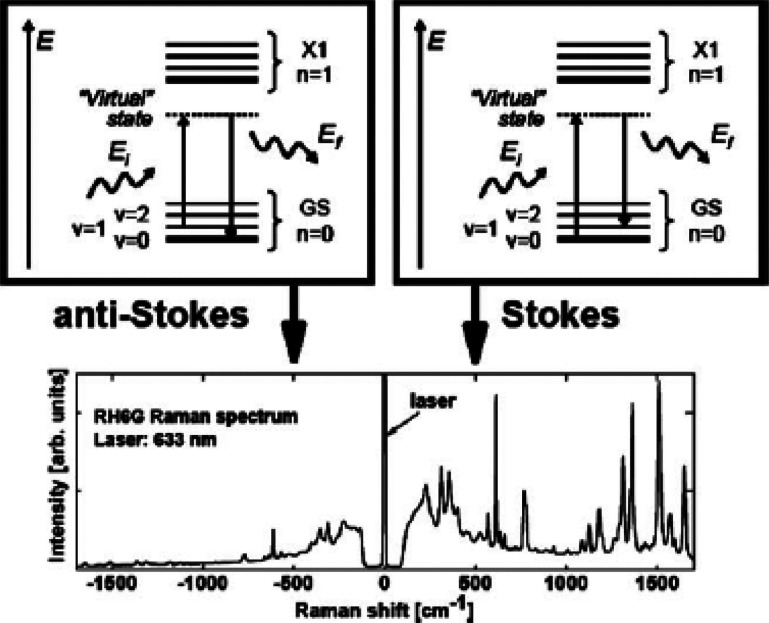
Schematic (Jablonski) diagrams (top) showing the basic elements of the electronic structure of a molecule. In quantum mechanical terms, a (nonresonant) Stokes process (right) consists of a transition to a virtual state, followed by a re-emission, leaving the molecule in the first (v = 1) vibrational excited state. This corresponds to positive Raman shifts in the spectrum. The anti-Stokes process (negative Raman shifts) on the top left follows the opposite path, thus producing a photon with a higher energy than that of the incoming one. The anti-Stokes process depends on the population of the excited level, while the Stokes process is (at low intensities where Raman stimulation is negligible) independent of this population. The processes depicted in the top-right (top-left) diagram contribute to the Stokes (anti-Stokes) part of the spectrum at lower (higher) energies with respect to the laser, as depicted in the bottom figure. Reproduced with permission from ref (176). Copyright 2008 Royal Society of Chemistry.