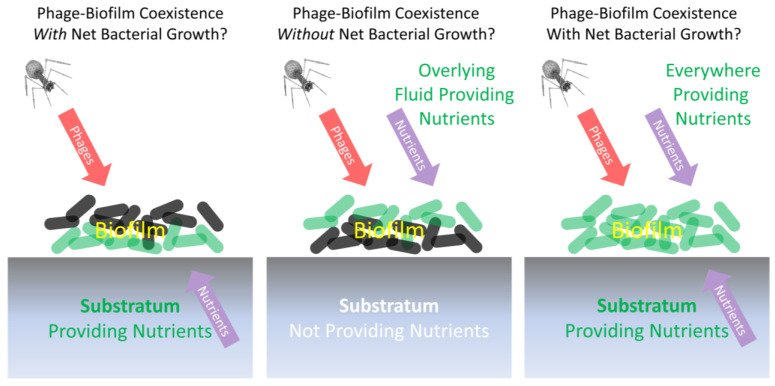
Figure 4.
Different scenarios for biofilms limiting (left and right) or not limiting (middle) net phage exploitation despite substantial ongoing bacterial replication. Those bacteria that are more metabolically active are shown as green (bottom layer on the left, top layer in the middle, and all layers on the right), while less metabolically active bacteria are illustrated as a dark gray. If nutrients and phages come from different spatial directions (left; the Eriksen et al. [80] experimental model), then ongoing bacterial growth may be possible despite biofilm exposure to phages. If phages and nutrients come from the same direction (middle; inert substratum model), then ongoing bacterial growth may be more difficult to sustain given this phage exposure. This middle example, however, is not from Eriksen et al. [80] but instead shares similarities with the experimental model of Darch et al. [44]. We can speculate that any survival of genetically phage-sensitive bacteria in this middle example might be associated with less metabolically active stationary-phase-like bacterial physiologies (Section 2.2), thereby resulting in a lack of net bacterial growth especially as phages eliminate surface bacteria that had possessed greater access to nutrients (as considered in Box 2). If nutrients are available everywhere, all of the time (right; the Eriksen et al. [80] theoretical model), then for a microcolony to survive, it must be large enough that bacteria can replicate exponentially faster than phages can reach and infect them. Assumed in all cases is that the biofilms in question do not possess additional mechanisms of inhibition of phages that would allow net bacterial replication despite phages and nutrients reaching those bacteria from the same direction.