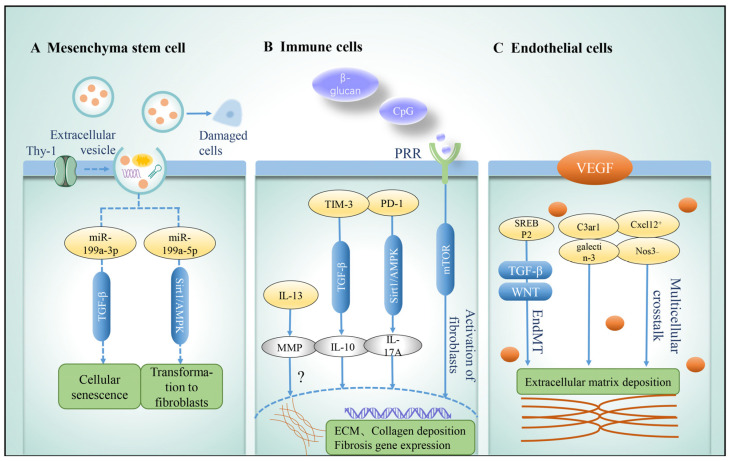
Figure 5.
Role of niche cells in idiopathic pulmonary fibrosis. Niche cells play different roles in the occurrence and development of idiopathic pulmonary fibrosis. (A) MSC-derived extracellular vesicles (mEV) produce a variety of growth factors and genetic material, transfer to damaged cells to repair cellular functions, and regulate intracellular signaling pathways (TGF-β, Sirt1/AMPK) to play an immunomodulatory role. (B) Immune cells have bidirectional regulatory effects, and their derived cytokines (IL-13, IL-10, and IL-17A) can not only promote the development of fibrosis through the activation of TGF-β, Sirt1/AMPK, mTOR, and other pathways but also play a role in immune regulation, tissue repair, and degradation of ECM. (C) The role of endothelial cells is mainly reflected in barrier function and cell communication. VEGF promotes lung repair after injury, but its different subtypes have different regulatory effects on IPF. For example, subgroups with high expression of Cxcl12 and low expression of Nos3 show pro-fibrosis phenotype. Endothelial–mesenchymal transition is an important process in the development of fibrosis. In this process, C3ar1 and galectin-3 are the key genes, TGF and Wnt signals are activated, ECM deposition is increased, and pulmonary fibrosis is aggravated. Abbreviations: TGF-β, transforming growth factor-β; ECM, extracellular matrix; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; VEGF, vascular endothelial growth factor; EndMT, endothelial–mesenchymal transition; PRR, pattern recognition receptors; MMP, matrix metalloproteinases; TIM-3, T-cell immunoglobulin domain and mucin domain-3; PD-1, programmed cell death 1; SREBP2, sterol regulatory element-binding protein 2; C3ar1, C3a receptor 1; Cxcl12, C-X-C motif chemokine ligand 12; Nos3, nitric oxide synthase 3.