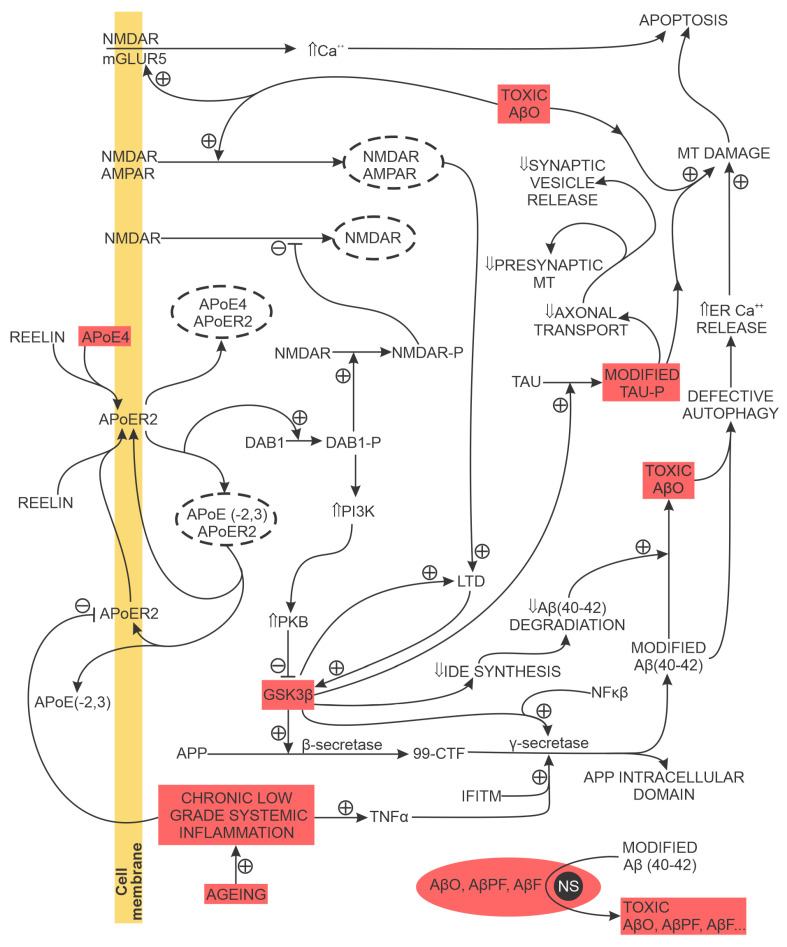
Figure 4.
Toxic soluble AβO and truncated, misfolded, and hyperphosphorylated toxic tau oligomers are the critical drivers of synapse loss and neuronal death in AD. The transformation of normal tau to its toxic oligomers is promoted by increased GSK3β activity; GSK3β inhibits Aβ42 degradation and promotes Aβ42 production by stimulating β- and γ-secretase activity. Increased TNFα and IFITM levels also promote high GSK3β activity. Ageing-associated chronic low-grade sterile inflammation and reduced reelin synthesis facilitate Aβ metabolism dysregulation. The secondary nucleation processes further accelerate the conversion of Aβ42 peptides to toxic AβOs at the AβO, protofibril and fibril nucleation sites. Abbreviations: ⇑ (increased), ⇓ (decreased), + (stimulating effect), − (inhibitory effect), - - - - (dashed lines denote internalised molecules), 99-CTF (99-amino acid membrane-bound C-terminal fragment), Aβ42 (an amyloid β peptide with 42 amino acid residues), AβO (amyloid β oligomers), AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor), APP (amyloid precursor protein), AβF (amyloid β fibril), AβPF (amyloid β protofibril), APOE (apolipoprotein E), APOER2 (reelin apolipoprotein E receptor 2), DAB1 (DAB adaptor protein 1), DAB1-P (phosphorylated DAB adaptor protein 1), GSK3β (glycogen synthase kinase 3β), IDE (insulin-degrading enzyme), IFITM (interferon-induced transmembrane protein), mGLUR5 (metabotropic glutamate receptor 5), NFκB (nuclear factor kappa-light-chain-enhancer of activated B cells), NMDAR (N-methyl-D-aspartate receptor), NS (nucleation site), PI3K (phosphoinositide 3-kinase), PKB (protein kinase B), Tau-P (truncated, misfolded, and hyperphosphorylated tau), TNFα (tumour necrosis factor α).