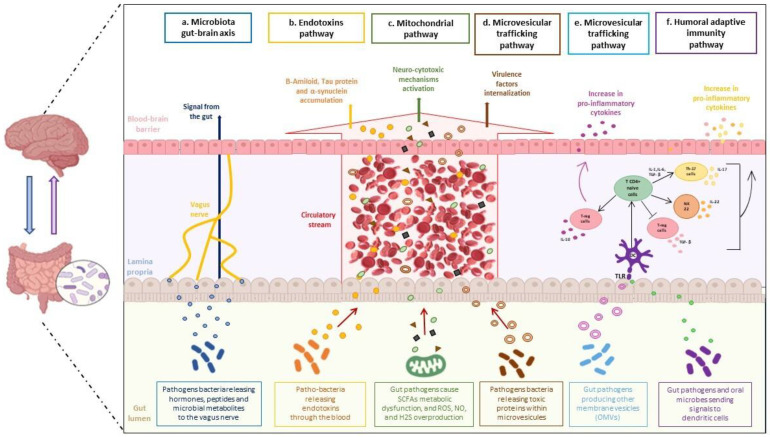
Figure 1.
The gut microbiota influences the onset of neurodegenerative diseases. Cooperation between a dysbiotic gut and the central nervous system occurs through several pathways: (a) the gut-brain axis, (b) endotoxins pathway, (c) mitochondrial pathway, (d,e) microvesicular trafficking pathway, and (f) humoral adaptive immunity pathway. The microbiota gut-brain axis (a): pathogenic gut bacteria release hormones and microbial metabolites that reach the brain via the vagus nerve, crossing the blood-brain barrier and inducing neurodegeneration processes. Endotoxins pathway (b): endotoxins reach the brain through the bloodstream and cause the accumulation of amyloid β, Tau-protein, and α-synuclein, modulating neuropathology. Mitochondrial pathway (c): intestinal pathogens cause SCFAs metabolic dysfunction and increased levels of ROS, NO, and H2S; these altered molecules reach the brain through the systemic circulation and induce activation of neuro cytotoxic mechanisms. Microvesicular trafficking pathway (d): pathogenic bacteria release toxic proteins within microvesicles that cross the blood-brain barrier through the bloodstream; this event allows internalization of virulence factors; (e) Gram-negative and positive bacteria produce vesicles from their membranes; these vesicles are internalized into dendritic cells and induce the differentiation of T-regulatory cells to produce IL-10, to create a neuroinflammatory environment. Humoral adaptive immunity pathway (f): gut microbes internalize pathogenic signals to dendritic cells; differentiation of Th-17 cells and NK-22 cells, and the pro-inflammatory cytokines, as IL-17 and IL-22,occur accordingly.