Figure 3.
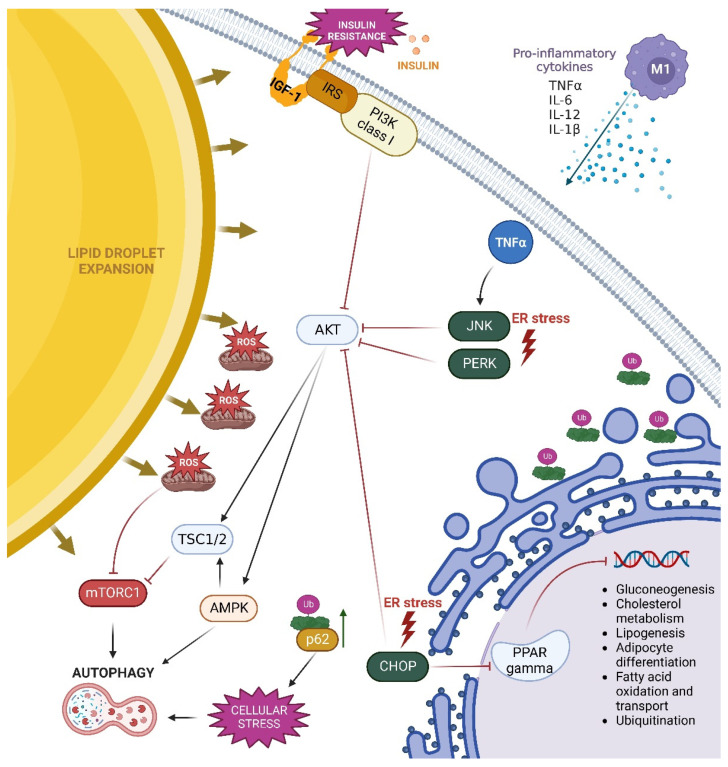
Pathways of autophagy activation in hypertrophic adipocytes in obesity. Enlargement of lipid droplets in hypertrophic adipocytes impairs mitochondrial oxidation and endoplasmic reticulum (ER) functions. Increased fatty acid content accelerates the process of beta-oxidation in obesity, resulting in mitochondrial dysfunction, elevated ROS production, oxidative stress, inflammatory cytokine secretion, and compromised ER function. These changes activate the ER-stress sensors PERK, JNK, and CHOP, which enhance autophagy by inhibiting AKT kinase. Additionally, increased TNF-α concentration enhances JNK-mediated pathway and inhibits AKT activity. Increased ROS production has an immediate effect on mTORC1, limiting its function and activating the autophagy process. Insulin resistance occurring in obesity also leads to diminished activation of the PI3K/AKT, which in turn promotes activation of TSC1/2 and AMPK. AMPK directly promotes autophagy, whereas TSC1/2 is involved in autophagy induction by inhibiting the mTORC1 complex. Furthermore, increased CHOP expression promotes PPAR inhibition. Active PPAR is involved in gluconeogenesis, cholesterol metabolism, lipogenesis, adipocyte differentiation, fatty acid oxidation and transport, and ubiquitination. In obesity, all of these processes are disrupted.