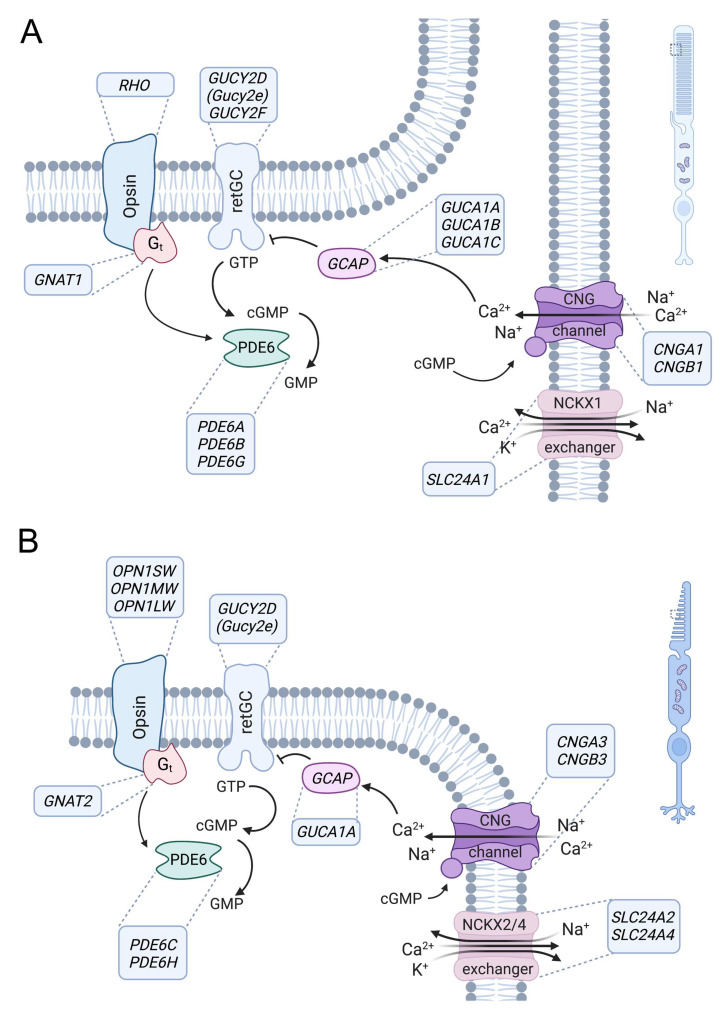
Figure 2.
Role of CNG channels in rod and cone photoreceptor signaling. Phototransduction in outer segments of rod (A) and cone (B) photoreceptors. The principle of the phototransduction is similar in both cell types, but key proteins are encoded by distinct but homologous genes (human gene names are indicated in the boxes next to the proteins). In the dark, the cyclic nucleotide-gated (CNG) channel (CNGA1/B1 in rods and CNGA3/B3 in cones) of the outer membrane is kept open by high concentrations of cyclic guanosine monophosphate (cGMP) produced by retinal guanylyl cyclase (retGC) (note: GUCY2E is a pseudogene in humans, whereas Gucy2e is functional in rodents and the major retGC encoding gene). The resulting influx of Na+ and Ca2+ depolarizes the plasma membrane. Light activates the opsin, which in turn activates transducin (Gt), whose alpha subunit activates a phosphodiesterase (PDE6) that leads to hydrolysis of cGMP. The decrease in the cGMP concentration leads to the closure of the CNG channel, resulting in membrane hyperpolarization. Ca2+ is an important regulator of phototransduction. At high concentrations, Ca2+ binds to guanylyl-cyclase-activating proteins (GCAP), leading to the inhibition of retGC. High Ca2+ concentrations also lead to a slight reduction in the cGMP affinity of the CNG channel through Ca2+/calmodulin-mediated feedback inhibition (not illustrated). Ca2+ is cleared from the outer segment via a Na+-Ca2+-K+-exchanger (NCKX1 in rods, NCKX2/4 in cones). At low Ca2+ levels, Ca2+-free GCAPs can bind and activate retGC to stimulate cGMP production and reopen the CNG channel.