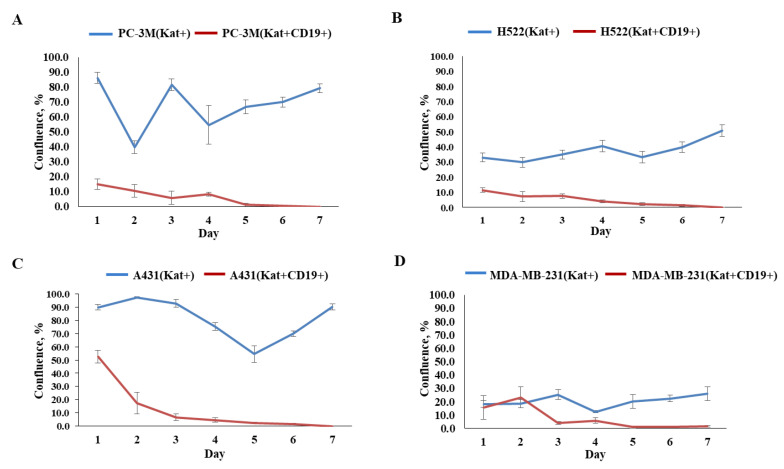
Figure 10.
Graphs representing comparative evaluation of CAR-T cells’ efficacy against modified tumor cells over 7-day period. (A)—comparative confluence of PC-3M(Kat+) and PC-3M(Kat+CD19+) cells after addition of CAR-T cells; (B)—comparative confluence of H522(Kat+) and H522(Kat+CD19+) cells after addition of CAR-T cells; (C)—comparative confluence of A431(Kat+) and A431(Kat+CD19+) cells after addition of CAR-T cells; (D)—comparative confluence of MDA-MB-231(Kat+) and MDA-MB-231(Kat+CD19+) cells after addition of CAR-T cells. The results are presented as mean, the error bars indicate standard deviation (n = 3, p < 0.05). Control tumor cells are shown in blue, modified CD19+ tumor cell lines in red.