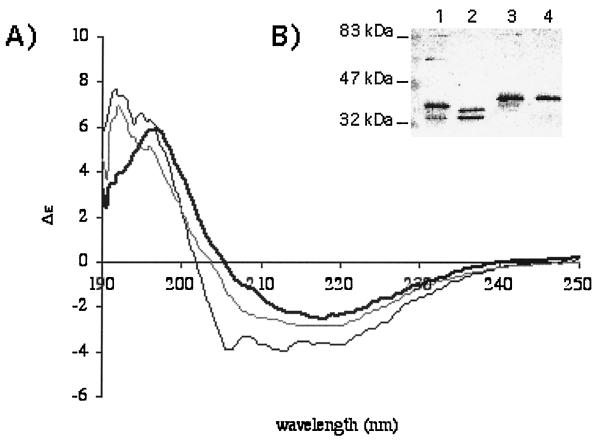
FIG. 7.
(A) Circular dichroim spectroscopy of OmpF, the archetype of porins purified from E. coli (bold line), refolded B. melitensis 16M Omp2b (gray line), or Omp2a (black line). All three proteins show a very high content of β-strands, although Omp2a is comparatively richer in the α-helix component. These spectra suggest a correct recovery of secondary structure for the refolded Brucella porins. (B) Western blot with purified Brucella porins (lane 1, B melitensis 16M Omp2b; lane 2, B. abortus 45/20 Omp2b; lane 3, B. ovis 76-250 Omp2b; lane 4, B. suis 83-210 Omp2b) and detection with a mix of MAbs directed against Omp2b. Purified trimers are unstable in the presence of SDS, and several quaternary states are visible: trimers at about 90 kDa, dimers, and several forms of monomers. The refolded Omp2b size variants, with the exception of that of B. suis 83–210, show a pattern with trimeric state, suggesting that correct refolding also occurred for the variant porins, although quantification was not possible with this experiment.