Figure 4.
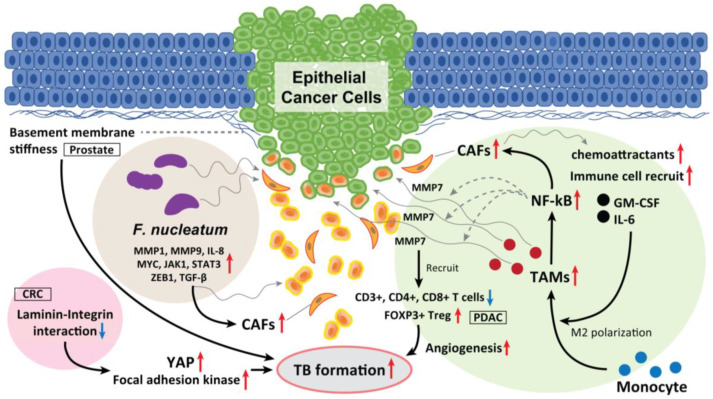
The role of TAM, laminin–integrin interaction, basement membrane stiffness, and F. nucleatum as a TB driver. TAMs located in the surrounding of the tumor mass induce loss of tight junction proteins at tumor cell–cell contacts, and cause TB from the colonosphere bulk of CRC. This is due to the MMP7 secretion by activating the NF-κB pathway. Moreover, CAF-derived exosomes serve as chemoattractants, which recruit various immune cells, including monocytes, promoting CRC progression and the release of cancer cell-derived exosomes. In PDAC, high-grade TB cases display lower M1 macrophages in the stroma and increased M2 macrophages in the tumor tissue, and displayed fewer CD3+, CD4+, and CD8+ T cells. Inversely, FOXP3+ Tregs were found to be elevated in high-grade TB cases. CAFs also recruit granulocyte-macrophage colony-stimulating factor and IL-6, promote the differentiation of monocytes into M2 macrophages and activate them to release chemokines and exosomes, which promote TB formation. Softening and enhanced remodeling of the basement membrane also promote TB development in stratified epidermis via the activation of focal adhesion kinase and YAP, while stiffening of the basement membrane promotes folding, and the laminin–integrin crosstalk in the basement membrane plays a key role to generate TBs. Moreover, F. nucleatum upregulated the expression of p-EMT-related genes in HNSCC cells with an epithelial phenotype. F. nucleatum-infected HNSCC cells had upregulated MMP1, MMP9, and IL-8. The expression of cell survival markers MYC, JAK1, and STAT3 and EMT markers ZEB1 and TGF-β were also significantly elevated and promoted TB development. These mediators also recruit CAFs in the TME and promote TB formation.