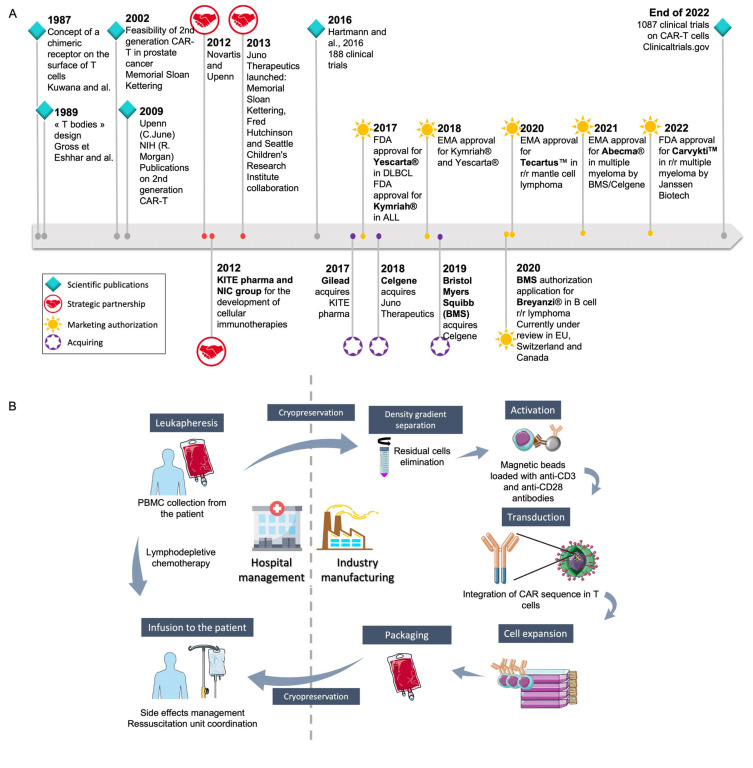
Figure 1.
Generalities on CAR-T cell development and their industrial and hospital management. (A) The way to marketing authorization of the different available CAR-T cells: academic discoveries led either (i) to start-up creation (Juno Therapeutics, Celgene, Kite pharma) to allow the development of clinical trials, before getting acquired by pharmaceutical companies or (ii) to the licensing to a pharmaceutical company. CAR: Chimeric Antigen Receptor; DLBCL: Diffuse Large B-Cell Lymphoma; EMA: European Medicines agency; FDA: Food and Drug Administration; NIC: National Cancer Institute; NIH: National Institutes of Health; r/r: relapsed refractory [2,10,11] (B) The logistic to allow the production of autologous CAR-T cells by pharmaceutical companies. Unlike other medicinal products, the raw material is specific to each CAR-T cell production. This required the participation of academic centers on crucial step such as collection of the leukapheresis or preservation of the ATMP and thawing before infusion into the patient. Different steps are not under the pharmaceutical company control, unless an audit validates the compliance with the company procedures.