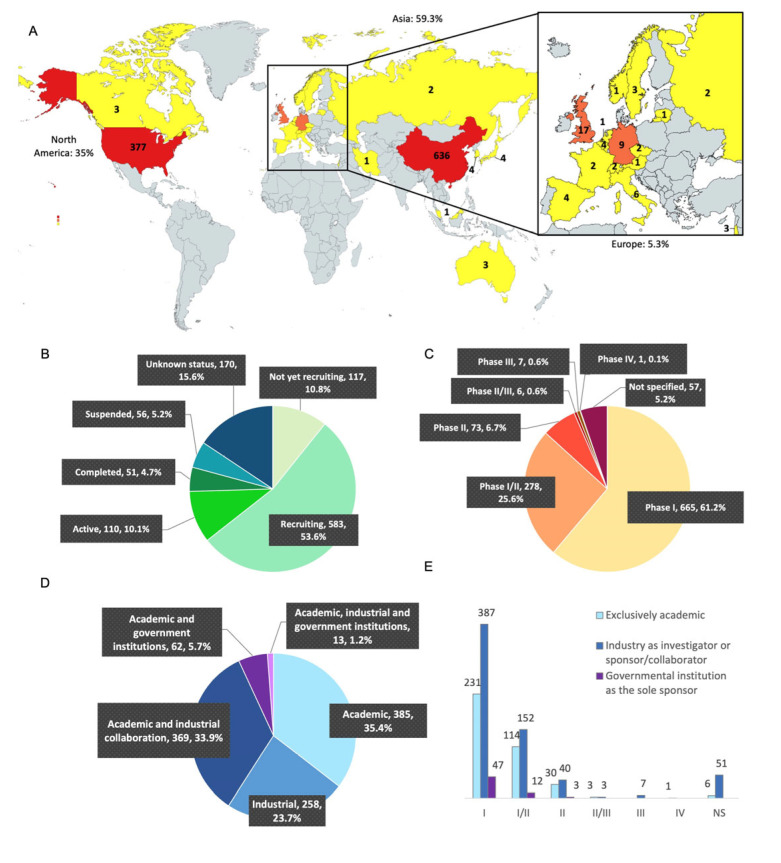
Figure 2.
Distribution of CAR-T cell clinical trials in the world in 2022. (A) Geographical distribution: The United States and China dominate in number of studies, with 377 (34.7%) and 636 (58.5%) studies, respectively. North America is also represented by Canada (3 studies). Apart from China, the Asian countries have 9 studies (0.8%) with Japan (4), South Korea (4), Malaysia (1) and Iran (1). Europe has 58 studies (5.3%): UK (17), Germany (9), Italy (6), Belgium (4), Spain (4), Sweden (3), Israel (3), Switzerland (2), Czech Republic (2), Russia (2), France (2), Lithuania (1), Netherlands (1), Austria (1), and Finland (1). Mapchart.net. (B) Status distribution: 117 studies have not started the recruitment (10.8%), 583 are currently recruiting patients (53.6%), 110 are active (10.1%), 51 have been completed (4.7%), 56 have been discontinued (5.2%), and 170 have unknown status (15.6%). (C) Study phases: 665 studies are in phase I (61.2%), 278 in phase I/II (25.6%), 73 in phase II (6.7%), 6 in phase II/III (0.6%), and 7 in phase III (0.6%). One study is in phase IV (0.1%) and 57 studies did not mention the information (5.2%). NS = not specified. (D) Distribution of clinical trials according to investigators and collaborators: out of 1087 clinical trials, 385 are mentioned only with an academic institution (35.4%), 258 are carried out only by a pharmaceutical industry (23.7%), 369 are carried out by an academic center in collaboration with an industrial company (33.9%), 62 are carried out by an academic center in collaboration with a governmental institution such as the NIH (5.7%), and finally 13 of the trials are carried out by a collaboration between these three actors (1.2%). (E) Distribution of clinical trials according to investigators and collaborators: number of academic clinical trials or in collaboration with an industry or in collaboration with a governmental institution according to the phases. The trials led by the three actors at the same time were added to the industrial collaboration group.