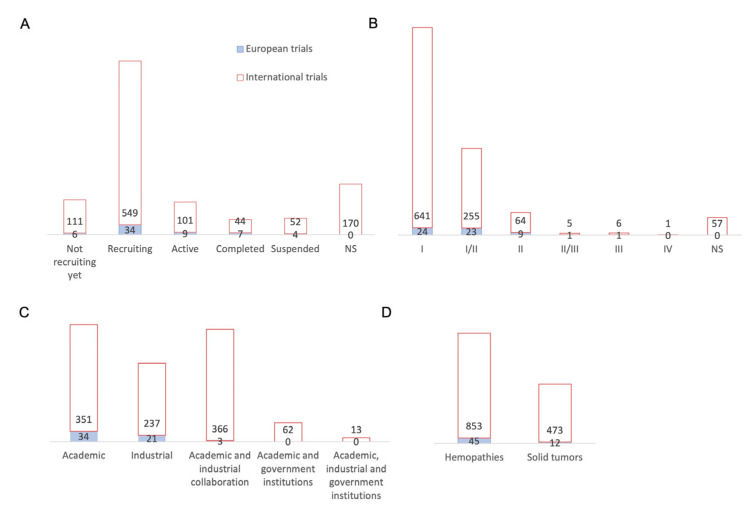
Figure 3.
Comparison of European CAR-T cell clinical trials compared with international trials. (A) Status distribution: 6 studies have not started the recruitment, 34 are currently recruiting patients, 9 are active, 7 have been completed, and 4 have been discontinued. (B) Study phases: 24 studies are in phase I, 23 in phase I/II, 9 in phase II, 1 in phase II/III, and 1 study in phase III. (C) Distribution of clinical trials according to investigators and collaborators: out of 58 clinical trials: 34 are carried out by an academic center, 21 are carried out by a pharmaceutical industry, and 3 are carried out by an academic center in collaboration with an pharmaceutical company. (D) Distribution of conditions: hematological malignancies were studied in 45 European studies and solid tumors in 12 European studies. If we compare with international trials outside Europe, we can see that hematological malignancies were studied in 853 studies and solid tumors in 473 studies. In this graph, studies can target multiple conditions, which explains the greater number of conditions investigated compared to the number of studies. NS = not specified.