Table 2. Generality of the Michael Reaction of 1 and 2a.
| entry | R | time (h) | yieldb (%) | eec (%) |
|---|---|---|---|---|
| 1 | Phenyl (2a) | 3 | 69 | 95 |
| 2 | p-MeO-C6H4 (2b) | 24 | 50 | 95 |
| 3 | p-Br-C6H4 (2c) | 2 | 71 | 94 |
| 4 | m-Br-C6H4 (2d) | 2 | 75 | 95 |
| 5 | o-Br-C6H4 (2e) | 2 | 78 | 95 |
| 6 | 2-furyl (2f) | 30 | 76 | 94 |
| 7 | SiMe2Ph (2g) | 12 | 69 | 98 |
| 8 | Me (2h) | 24 | 55 | 76 |
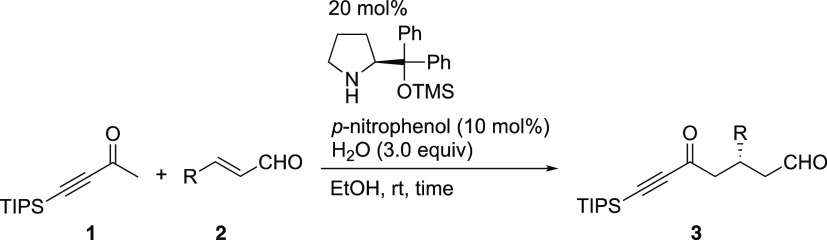
Reactions were performed by employing α,β-unsaturated aldehyde 2 (0.18 mmol), ketone 1 (0.15 mmol), organocatalyst (0.030 mmol), water (0.45 mmol), and p-nitrophenol (0.015 mmol) in EtOH (0.60 mL) at room temperature for the indicated time.
Isolated yield.
Enantiomeric excess (ee) of the products, as determined by HPLC analysis over a chiral solid phase after conversion to α,β-unsaturated ester by the treatment with Ph3P = CHCO2Et.