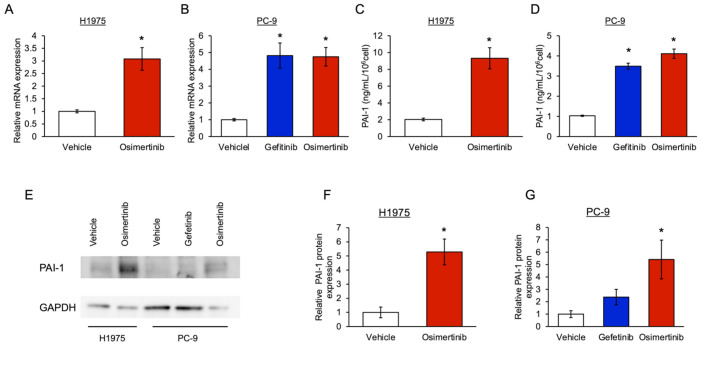
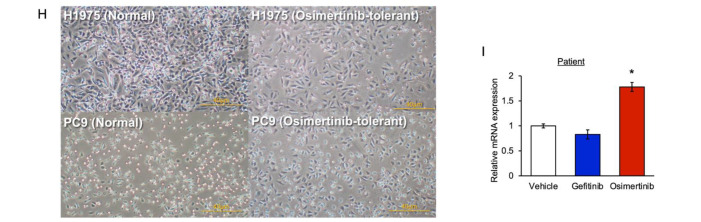
Figure 1.
PAI-1 expression in EGFR-TKI-tolerant cancer cells. (A,B) qRT-PCR analysis of the expression of PAI-1 mRNA in EGFR-TKI-tolerant PC-9 and H1975 cells compared with the corresponding mRNA expression in the vehicle controls. Relative expression level of PAI-1 is shown with standard error bars (n = 3); * p < 0.05 compared with cells exposed to vehicle controls (Student’s t-test or one-way ANOVA with Tukey’s test). (C,D) Concentration of PAI-1 protein in the culture medium of EGFR-TKI-tolerant PC-9 and H1975 cells compared with the PAI-1 protein concentration seen in the controls. Mean concentrations are shown with standard error bars (n = 5); * p < 0.05 compared with cells exposed to vehicle controls (Student’s t-test or one-way ANOVA with Tukey’s test). (E) Western blotting of PAI-1 in EGFR-TKI-tolerant PC-9 and H1975 cells compared with the vehicle controls. (F,G) Levels of PAI-1 protein normalized to GAPDH in EGFR-TKI-tolerant PC-9 and H1975 cells compared with the vehicle controls. The relative expression level of PAI-1 is shown with standard error bars (n = 3); * p < 0.05 compared with cells exposed to the vehicle controls (Student’s t-test or one-way ANOVA with Tukey’s test). (H) Micrographs of normal and osimertinib-tolerant cells. Upper-left: normal H1975, upper-right: osimertinib-tolerant H1975 cells; lower-left: normal PC-9 cells; lower-right: osimertinib-tolerant PC-9 cells; bar: 40 µm. (I) qRT-PCR analysis of the expression of PAI-1 mRNA in EGFR-TKI-tolerant and control cancer cells derived from a patient with an EGFR exon 19 deletion and the T790M mutation seven months after afatinib administration. As in the other cell experiments, gefitinib and osimertinib were administered. The relative expression level of PAI-1 is shown with standard error bars (n = 3); * p < 0.05 compared with cells exposed to vehicle controls (one-way ANOVA with Tukey’s test). All the whole western blot figures can be found in the Supplementary Materials (Figure S8).