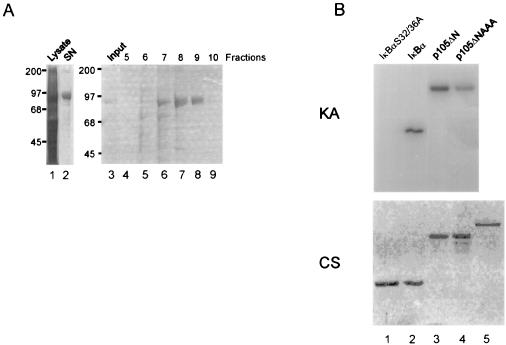
FIG. 1.
Purified recombinant IKKβ phosphorylates the signal response domain of p105 directly. (A) Coomassie brilliant blue-stained SDS-PAGE showing the purification of IKKβ. His-tagged IKKβ was expressed in SF9 cells with the baculovirus system. IKKβ protein from the lysates of infected cells (lane 1) was bound to Ni+ -agarose and cleaved off by thrombin digestion (lane 2). The supernatant was bound in 50 mM Tris (pH 7)–1 mM DTT–1 mM EDTA to a MonoQ column and eluted with a 10-ml gradient of 0 to 1 M NaCl (lanes 4 to 9). The protein eluted at about 500 mM NaCl. Sizes are shown in kilodaltons. (B) The purified protein (fraction 9) (lane 5, lower panel) was used in an in vitro kinase assay with IκBα S32/36A, wild-type IκBα, p105ΔN, and p105ΔNAAA, as indicated. p105ΔNAAA contains serine-to-alanine mutations at amino acids 921, 923, and 932 and a serine-to-threonine mutation at residue 927. Top, kinase assay (KA); bottom, Coomassie brilliant blue-stained (CS) SDS-PAGE of IκBα, p105, and IKKβ proteins.