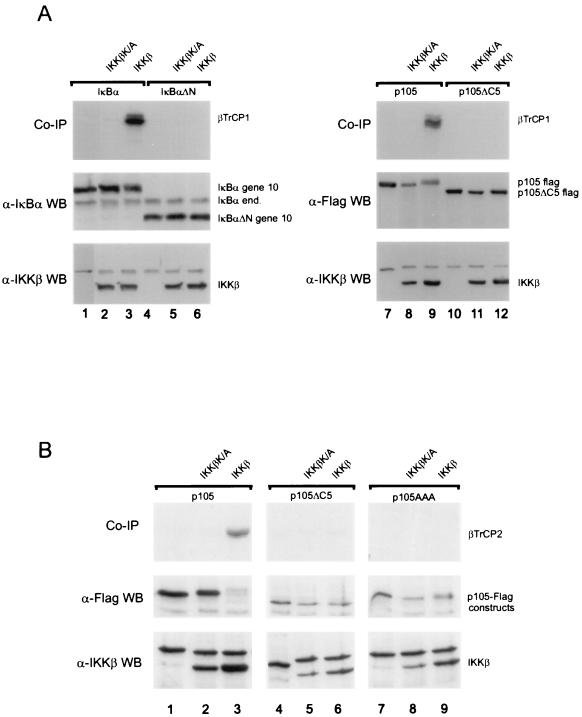
FIG. 3.
SCF ligase receptor subunits βTrCP1 and βTrCP2 recognize IKK-phosphorylated p105. (A) Plates of 293 cells were transfected with 1 μg of IκBα (lanes 1 to 3), 1 μg of IκBαΔN (lanes 4 to 6), 2 μg of p105 (lanes 7 to 9), or 2 μg of p105ΔC5 (lanes 10 to 12) together with kinase inactive IKKβ (lanes 2, 5, 8, and 11) or wild-type IKKβ (lanes 3, 6, 9, and 12). Cells were extracted with 200 mM NaCl–50 mM HEPES (pH 7.5)–0.5% NP-40–1 mM EDTA–10% glycerol. The extracts were diluted to 100 mM NaCl–50 mM HEPES (pH 7.5)–0.5% NP-40–1 mM EDTA– 5% glycerol and incubated for preclearance and immunoprecipitation with 4.5 μl of in vitro-translated, 35S-labeled βTrCP1-HA. Immunoprecipitations were performed with anti-gene 10 (IκBα) or anti-Flag (p105) antibodies. Coprecipitated βTrCP1 (top panels), expression (Western blots) of IκBα constructs and endogenous IκBα (anti-IκBα antibody; middle panel, left), of p105 constructs (anti-Flag antibody, right panel) as well as of expressed wild-type and mutant IKKβ are shown as indicated. (B) A similar experiment was performed using p105 (lane 1 to 3) p105ΔC5 (lanes 4 to 6) and p105AAA (lanes 7 to 9) expression constructs cotransfected with IKKβ K/A (lanes 2, 5, and 8) or IKKβ (lanes 3, 6, and 9). Cell lysis and immunoprecipitation were performed as described above, using anti-Flag antibodies and in vitro translated βTrCP2-HA/Hos protein.