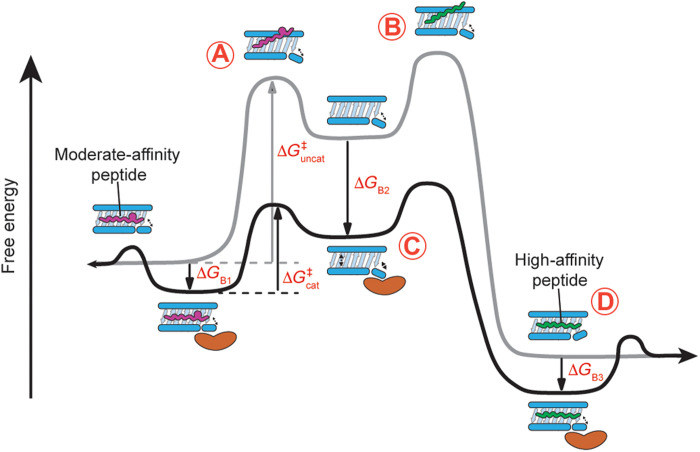
Fig. 8. TAPBPR orthologs can enact multiple mechanisms to enable ligand exchange on MHC-I molecules.
(A) MHC-I loaded with a moderate affinity placeholder peptide can spontaneously but slowly generate empty molecules. (B) These empty molecules overcome a high-activation energy barrier (ΔG‡uncat) to be in the transient peptide-receptive state for peptide loading. (C) A catalytic amount of TAPBPR functions to lower the activation energy barrier (ΔG‡cat) by recognizing the empty molecules with high affinity (binding free energy ΔGB2) and stabilizing an open conformation for peptide loading. (D) Loading of high-affinity peptides induces a closed MHC-I groove conformation leading to TAPBPR dissociation due to the substantially reduced affinity of TAPBPR toward peptide-loaded molecules (indicated by binding free energies ΔGB1 and ΔGB3). The reaction coordinate diagram from left to right shows the exchange of a moderate-affinity peptide to a high-affinity peptide, which can happen in the opposite direction with a much higher activation energy barrier and thus slower kinetics.