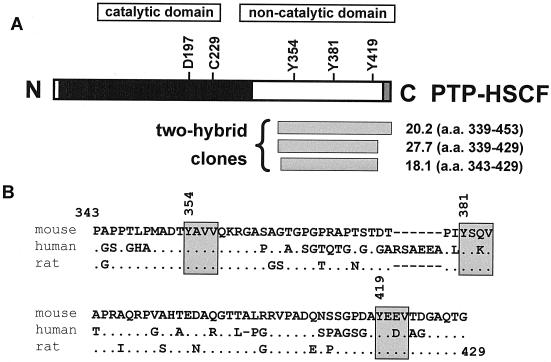
FIG. 1.
Identification of PTP-HSCF as a potential Csk-binding protein in the yeast two-hybrid system. (A) The primary structure of PTP-HSCF is shown. The positions of conserved critical residues in the phosphatase domain (aspartate 197 and cysteine 229 in the mouse protein) and of three tyrosines in the carboxy-terminal noncatalytic domain (tyrosines 354, 381, and 419) are indicated. The locations and boundaries of the three independent ptp-hscf cDNA clones identified in the yeast two-hybrid screen are shown at the bottom. a. a., amino acid. (B) Comparison of the carboxy-terminal sequences of mouse, human, and rat PTP-HSCF. The amino acid sequences (residues 343 to 429) corresponding to the smallest cDNA clone identified in the yeast two-hybrid screen (clone 18.1) are compared for mouse, human, and rat PTP-HSCF. In the case of the human protein sequence, part of the data is derived from expressed sequence tag database analyses. Identical amino acids are shown as dots, while gaps in the sequence alignment are revealed by hyphens. The three conserved tyrosine-based motifs are boxed.