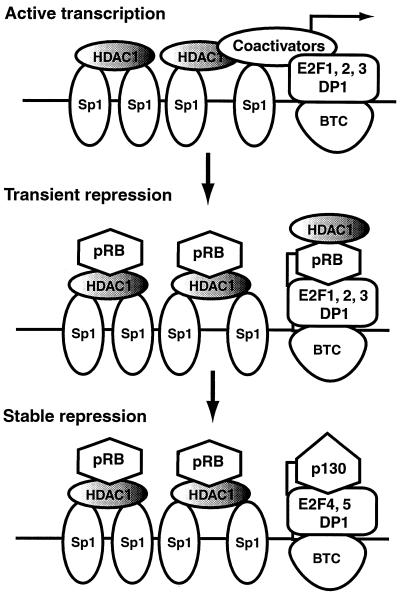
FIG. 12.
A dynamic model for cooperative repression of the dhfr promoter by Sp1 and E2F. In cycling cells, Sp1 complexes and complexes of and E2F-1 to -3 with DP drive transcription of the dhfr gene. During the first 24 h of serum withdrawal, dephosphorylation of pRb results in the binding of pRb to Sp1-HDAC1 complexes and complexes of E2F-1 to -3 with DP, thereby dampening transcription. During the initial phases of serum deprivation, the dhfr promoter is sensitive to TSA, suggesting that HDAC activity associated with pRb is involved in repression at this time. After 24 h a TSA-insensitive state that correlates with the expression of p130 and E2F-4 is achieved, suggesting that E2F-4–DP–p130 complexes cooperate with Sp1-HDAC1-pRb complexes to establish stable repression of the promoter. Upon growth stimulation, repression is relieved by phosphorylation of pRb and p130 at the G1 restriction point. Activation occurs thereafter by recruitment of complexes of E2F-1 to -3 with DP and associated coactivators to the E2F sites. HDAC1 activity may be regulated by association with pRb or other mechanisms. The model posits that Sp1 and E2F cooperate in a dynamic fashion during the establishment of repression, relief of repression during G1, and activation of transcription after the G1 restriction point. Based on genomic footprinting experiments, the model suggests that a basal transcription complex (BTC) is present at the E2F sites throughout the cell cycle. The components of the BTC are not known.