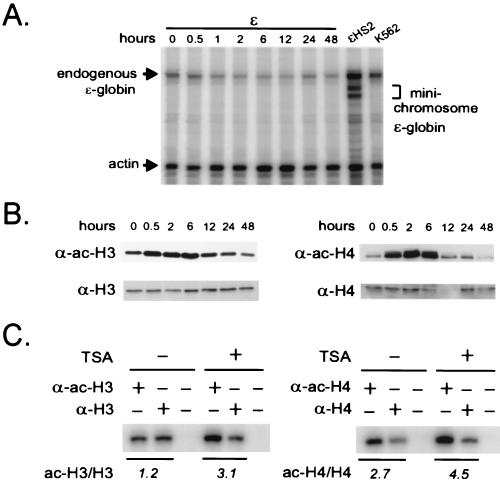
FIG. 5.
Global histone acetylation in the absence of HS2 is insufficient to activate the ɛ-globin gene. (A) RNase protection. A K562 clone carrying ɛ minichromosomes was treated with TSA (150 ng/ml) for 48 h. RNA was prepared at various time points and analyzed by an RNase protection assay as described in Materials and Methods. The position of a band protected by endogenous ɛ-globin RNA transcripts in K562 cell RNA is indicated, as are the positions of bands protected by minichromosomal ɛ-globin RNA in ɛHS2 RNA. Actin RNA served as the load control. (B) Western analysis. Histones were acid extracted from the cells treated for 0 to 48 h with 150 ng of TSA per ml, and 2.5 μg of the acid-extracted proteins was electrophoresed on an acrylamide gel, transferred to a nitrocellulose membrane, and sequentially probed with either anti-H3 and anti-acetylated-H3 antibodies (α-H3 and α-ac-H3, respectively) or anti-H4 and anti-acetylated-H4 antibodies (α-H4 and α-ac-H4, respectively). (C) ChIP. After treatment of K562 cells containing ɛ minichromosomes with 150 ng of TSA per ml for 6 h, nuclei were prepared and digested with MNase and immunoprecipitation was carried out with anti-H3 and anti-acetylated-H3 antibodies or with anti-H4 and anti-acetylated-H4 antibodies as described in the legend to Fig. 4. Coprecipitated DNA was detected by PCR amplification using the N1 primers (Fig. 2).