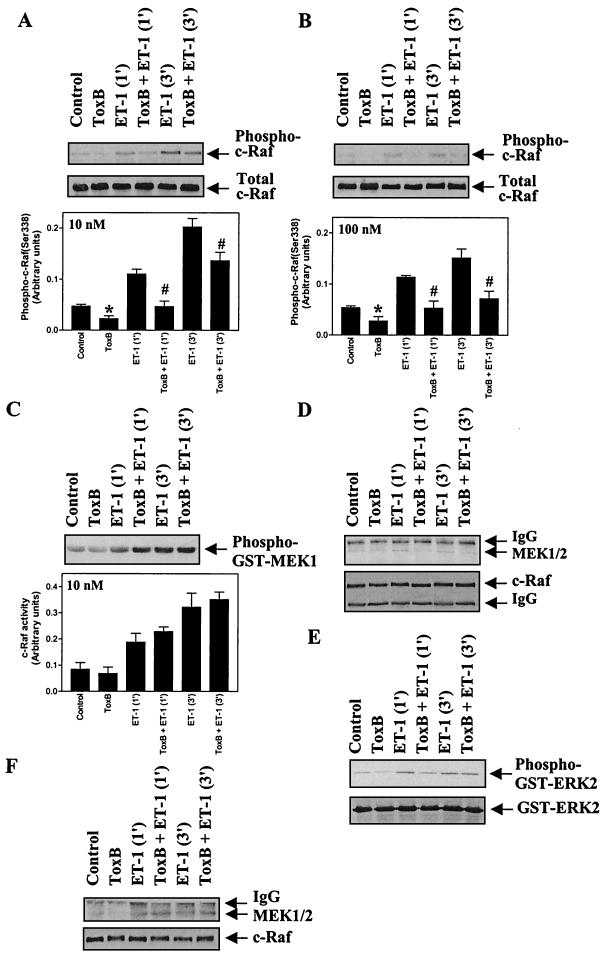
FIG. 6.
Toxin B inhibits c-Raf(Ser-338) phosphorylation but not c-Raf activity and attenuates MEK1 association with c-Raf in myocytes exposed to 10 nM ET-1. Myocytes were unstimulated or exposed to 10 nM ET-1 (A, C, D, and E) or 100 nM ET-1 (B and F) for the times indicated, and c-Raf was immunoprecipitated from myocyte extracts. (A and B) c-Raf immunoprecipitates were immunoblotted with antibodies selective for phospho(Ser-338)-c-Raf (upper blots) or total c-Raf (lower blots). Blots were analyzed by scanning densitometry (bar graphs). Results are means ± SEM for four separate myocyte preparations. ∗, P < 0.05, relative to unstimulated myocytes in the absence of toxin B (unpaired two-tailed t test). P < 0.05 relative to myocytes exposed to ET-1 in the absence of toxin B (unpaired two-tailed t test). (C) c-Raf activity was determined using GST-MEK1 as the substrate. Phosphorylation of GST-MEK1 was assessed by immunoblotting with antibodies selective for phospho-MEK1 and/or -2 (upper panel). Blots were analyzed by scanning densitometry (lower panel). Results are means ± SEM for three separate myocyte preparations. (D and F) c-Raf immunoprecipitates were immunoblotted for total MEK1/2 (upper panels) or for c-Raf (lower panels). (E) c-Raf immunoprecipitates were assayed for associated ERK-activating activity using GST-ERK2 as substrate. Phosphorylation of GST-ERK2 was assessed by immunoblotting with antibodies selective for phospho-ERK1 and -2 (upper panel). Parallel blots were probed with antibodies to total ERK1 and -2 (lower panel). The experiments were repeated with three separate preparations of myocytes with similar results. IgG, immunoglobulin G.