Abstract
Background
Long-term data on the effectiveness and safety of the booster dose of anti-SARS-CoV-2 vaccines in people affected by multiple sclerosis (pwMS) are lacking, hence a retrospective monocentric study exploring these issues was undertaken.
Materials and methods
PwMS who had received the booster dose of anti-COVID19 mRNA vaccines (either Comirnaty or Spikevax) according to the national regulation were included. The occurrence of adverse events or disease reactivation and SARS-CoV-2 infection were recorded up to last follow-up. Factors predictive of COVID-19 were explored using logistic regression analyses. A two-tailed p-value <0.05 was considered significant.
Results
One hundred and fourteen pwMS were included: 80 females (70%); median age at the booster dose 42 years (range 21 – 73); 106/114 patients (93%) were receiving a disease-modifying treatment at vaccination. The median follow-up after the booster dose was 6 (range 2 – 7) months. Adverse events were experienced in 58% of the patients, being mild to moderate in most cases; 4 reactivations of MS were observed, two of which occurring within 4 weeks after the booster. SARS-CoV-2 infection was reported in 24/114 (21%) cases, occurring a median of 74 days (5–162) after the booster dose and requiring hospitalisation in 2 patients. Six cases received direct antiviral drugs. Age at vaccination and time between the primary vaccination cycle and the booster dose were independently and inversely associated with the risk of COVID-19 (HR 0.95 and 0.98, respectively).
Conclusions
The administration of the booster dose in pwMS showed an overall good safety profile and protected 79% of the patients from SARS-CoV-2 infection. The observed association between the risk of infection after the booster dose and both younger age at vaccination and shorter interval period to the booster dose suggest that unobserved confounders, possibly including behavioural and social factors, play a relevant role in determining the individual propensity to get infected with COVID-19.
Keywords: Multiple sclerosis, COVID-19, SARS-CoV-2, mRNA, Vaccine, Booster
1. Introduction
Vaccination against SARS-CoV-2 has been widely used to prevent the complications of COVID-19 (Fiolet et al., 2021) and it has been administered worldwide, especially in vulnerable populations (tracker, 2022). The Italian Ministry of Health recognized people affected by multiple sclerosis (pwMS) as a category with “high frailty” mainly due to disability and potential immunodepression induced by disease-modifying therapies (DMTs) (Salute). Despite a lower incidence of SARS-CoV-2 infection was initially reported in pwMS compared with the general population (possibly due to the adoption of precautionary measures), higher rates of hospitalization were observed (Moreno‐Torres et al., 2021). Several risk factors for severe outcomes were suggested, being older age, presence of comorbidities and treatment with anti-CD20 therapies the most consistent across studies (Muñoz-Jurado et al., 2022; Pistor et al., 2022).
DMTs affect the immune system with different mechanisms and might interfere with the development of an effective immune response after infections or vaccination, each at a different extent according to the mechanism of action (Eisenberg et al., 2013). A reduced antibody-mediated response to vaccines was reported for DMTs inducing lymphopenia, such as fingolimod and B cell depleting therapies, with a reduction in the proportion of seroconversion and antibody titres compared to pwMS treated with other DMTs (Capone et al., 2022; Sormani et al., 2021; van Kempen et al., 2022). However, a robust T cell-mediated response to vaccination was observed in patients treated with CD20-depleting therapies (Apostolidis et al., 2021), likely contributing remarkably to protection against hospitalization or death (Moss, 2022).
Furthermore, the protective effect of vaccines tends to wane over time, as suggested by a progressive reduction in anti-SARS-CoV-2 antibody titres and increase in COVID-19 incidence (Alharbi et al., 2022; Fabiani et al., 2022; Mastroianni et al., 2022; Shrotri et al., 2022). For this reason, the administration of a booster dose of anti-SARS-CoV-2 vaccines was firstly studied in Israel and then approved worldwide after evidence of its effectiveness in healthy people (Tartof et al., 2022).
PwMS were vaccinated with priority with the booster dose of anti-SARS-CoV-2 mRNA vaccines, but only a few real-world data on the effectiveness and safety of the booster dose in this patient population are available so far, especially in the mid-long term follow-up (Dreyer-Alster et al., 2022).
In the present study, a real-world cohort of pwMS receiving different DMTs who had previously completed the primary vaccination cycle and who received the booster dose was followed to assess the occurrence of adverse events and SARS-CoV-2 infection.
2. Materials and methods
2.1. Study design
A retrospective monocentric study aimed at exploring the safety and effectiveness of the SARS-CoV-2 booster vaccine in pwMS who attended the Tuscan Region MS Referral Centre of the Careggi University Hospital in Florence, Italy.
2.2. Patient selection
PwMS diagnosed according to the McDonald criteria (McDonald et al., 2001; Polman et al., 2011) who were previously enroled in a retrospective study evaluating the safety and humoral response elicited by anti-SARS-CoV-2 vaccines (Mariottini et al., 2022) and who had thereafter received the booster dose according to the national regulation were included.
2.3. Clinical examinations and outcomes
Clinical-demographic information including age, gender, MS phenotype, disease duration, treatment duration, and disability (assessed as Expanded Disability Status Scale, EDSS) score (Kurtzke, 1983) was retrospectively collected from clinical records. The occurrence of adverse events of any type and SARS-CoV-2 infection following the booster administration were also investigated by dedicated phone interviews and recorded.
For MS reactivation, all the events observed during the follow-up were recorded, but only those occurring within 8 weeks from the booster administration were considered as possibly related to the vaccination.
The number and severity of COVID-19 infections occurring throughout the follow-up were used to estimate the effectiveness of the booster dose. Baseline characteristics of the patients, including treatment status and the type of DMTs received, were explored as potential predictors of SARS-CoV-2 infection. According to the national prescribing indications, DMTs were classified into first-line DMTs or second-line DMTs. First line DMTs encompassed all the followings: glatiramer-acetate, interferons, dimethyl-fumarate and teriflunomide. Second line DMTs included the following: natalizumab, fingolimod, cladribine, alemtuzumab and ocrelizumab. Azathioprine and rituximab, both licensed in Italy for the treatment of autoimmune diseases of the nervous system, were included in the first and second line DMTs classes, respectively. The effect of drugs with a depletive mechanism of action (i.e. ocrelizumab, rituximab, alemtuzumab and cladribine) was further explored comparing this class with not depleting treatments.
2.4. Statistical methods
Baseline characteristics of patients are reported as median and range or as number and frequency, as appropriate. Factors predictive of COVID-19 infection were explored using Cox analyses. Comparisons between groups were carried out using non-parametric tests (Mann-Whitney test for continuous and Chi-square test for dichotomic variables). The statistics software used was SPSS version 25 (Windows). A two-tailed p-value <0.05 was considered significant.
2.5. Data availability statement
Individual de-identified participant data will be shared upon written request.
3. Results
3.1. Patient characteristics
One hundred and fourteen MS patients were included. Baseline characteristics are summarized in Table 1 . Briefly, there were 80 females (70%); MS phenotype was relapsing-remitting (RR-) in 103 cases (90%) and secondary-progressive (SP-) in 11 cases. Most of the patients (106/114, 93%) were receiving a DMT at the time of vaccination, and 70% of the cases were treated with second-line DMTs (Table 2 ). Patients treated with anti-CD20 monoclonal antibodies were receiving a standard interval dosing at the time of the booster dose.
Table 1.
Clinical-demographic characteristics of the MS population included (n = 114).
| Median | (range) | |
|---|---|---|
| Age, years | 42 | (21 – 73) |
| Disease duration, years | 10 | (0 – 38) |
| Treatment duration since first DMT, years | 8 | (0 – 25) |
| Number of previous DMTs | 1 | (0 – 5) |
| EDSS | 1.5 | (0 – 7.5) |
| Number | (%) | |
| Gender, female | 80 | (70%) |
| MS form, RR | 103 | (90%) |
| MS form, SP | 11 | (8%) |
| On treatment | 106 | (93%) |
| Treatment with second line DMTs | 80 | (70%) |
| Treatment with depletive DMTs a | 37 | (32%) |
Depletive DMTs include all the following: rituximab, ocrelizumab, alemtuzumab, cladribine.
DMTs, disease-modifying treatments; EDSS, expanded disability status scale; MS, multiple sclerosis; RR, relapsing-remitting; SP, secondary progressive.
Table 2.
Disease-modifying treatment received at the time of vaccination.
| Cases treated | Duration of the treatment, months | |||
|---|---|---|---|---|
| N | (%) | Median | (range) | |
| Cyclophosphamide | 1 | (1%) | 25 | n.a. |
| Cladribine | 3 | (3%) | 28 | (18–32) |
| Fingolimod | 11 | (10%) | 72 | (25 - 118) |
| Glatiramer-acetate | 2 | (2%) | 37 | (15 - 59) |
| Interferons | 9 | (8%) | 117 | (27 - 162) |
| Natalizumab | 31 | (30%) | 48 | (12 - 139) |
| Ocrelizumab | 24 | (23%) | 26 | (2 - 61) |
| Rituximab | 10 | (9%) | 51 | (37 - 69) |
| Tecfidera | 13 | (12%) | 50 | (24 - 320) |
| Teriflunomide | 2 | (2%) | 36 | (30 - 42) |
The patients included received the booster dose a median of 193.5 (range 126 - 344) days after the last dose of the primary vaccination cycle. According to the national regulation, the booster consisted of the administration of either one dose of Comirnaty (Pfizer BioNTech BNT162b2 COVID-19 vaccine) (Agency) in 81 (71%) cases, or half-dose of Spikevax (COVID-19 Moderna mRNA −1273) (Agency) in the remaining 33 (29%) cases.
The median follow-up after the booster dose was 6 (range 2 – 7) months.
3.2. Adverse events or MS relapses following the booster dose
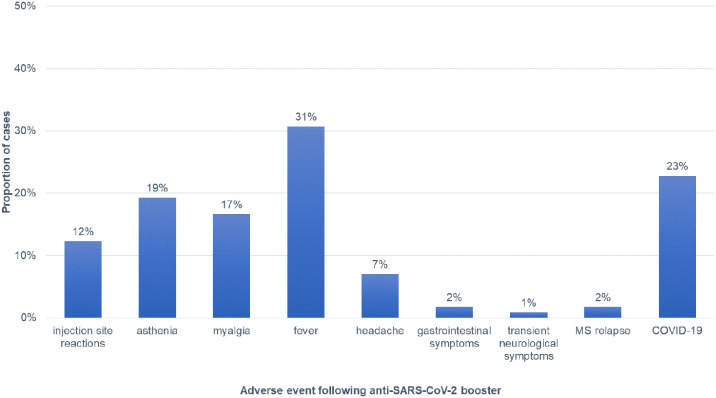
Adverse events were experienced by 66 patients (58%) and were mostly represented by fever (31%), asthenia (19%) and myalgia (17%). Further details on adverse events are reported in Fig. 1 .
Fig. 1.
Adverse events reported after the administration of the booster dose of anti-COVID19 mRNA vaccines.
MS relapse was observed in four cases (3.5%), and it occurred within 8 weeks after the booster administration in two cases. All these patients were affected by RR-MS with low disability (EDSS ranging from 1 to 2) and short disease-duration (median 9 years, range 3 – 14), and they were all receiving a DMT at vaccination (interferon, ocrelizumab, natalizumab, or fingolimod). Three out of four patients who relapsed were also infected by SARS-CoV-2, either before the vaccination (one case) or after the completion of the primary vaccination cycle (two cases).
MRI scans performed shortly after the relapse were available in two cases, showing at least one new enhancing lesion in both patients.
Two further patients had experienced a clinical relapse after the primary vaccination cycle but before the booster dose, as previously reported (Mariottini et al., 2022).
3.3. COVID-19 infection
SARS-CoV-2 infection was reported in 24/114 (21%) cases, as confirmed by nasal/oropharyngeal swabs with the detection of SARS-CoV-2 RNA or the virus antigen. The median time between the booster dose and the detection of a positive swab was 80.5 (range 5 – 262) days. The infection was symptomatic in 22/24 (92%) cases, and symptoms lasted for a median of 7 (range 2 - 30) days. The negativization of the nasal/oropharyngeal swab occurred after median 17.5 (range 7 – 42) days.
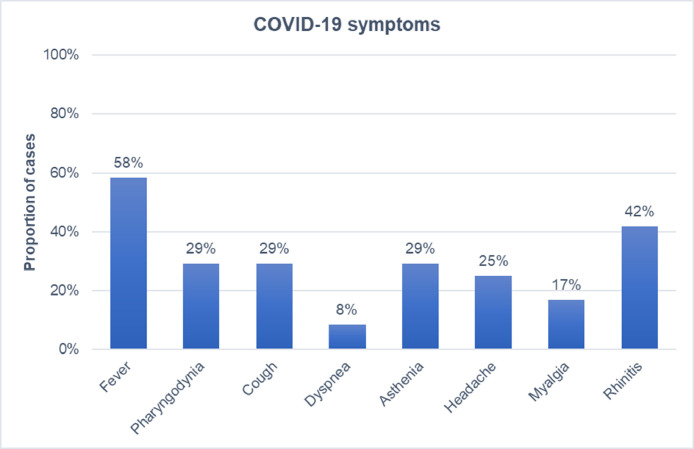
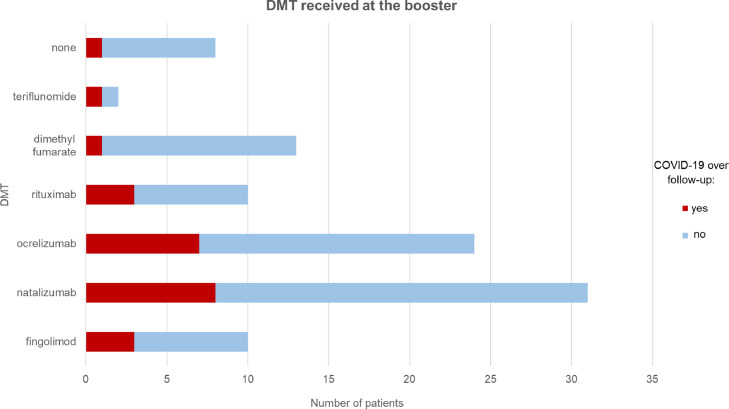
The most frequent symptoms were fever (58%), rhinitis (42%), cough and asthenia (29% each). Details on the remaining symptoms experienced during COVID-19 are summarized in Fig. 2 . Amongst the patients who tested positive for COVID-19, 8 were treated with natalizumab, 7 with ocrelizumab, 3 with fingolimod, 3 with rituximab, and one each with dimethyl-fumarate and teriflunomide; one patient was not receiving any DMTs (Fig. 3 ).
Fig. 2.
Proportion of MS cases who experienced each of the reported symptom during SARS-Cov2 infection (N = 24).
Fig. 3.
Patients who experienced SARS-CoV-2 infection (red) and those who did not (blue) stratified according to the disease-modifying treatment (DMT) received at the time of the booster administration. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Four patients who were treated with either ocrelizumab or natalizumab received monoclonal antibodies anti-SARS-CoV-2: casirivimab-imdevimab in three cases, and sotrovimab in one case. Remdesivir was administered in one case, whereas none received tocilizumab.
Two patients, one SP-MS female aged 38 years (EDSS 7; patient A) and one RR-MS female aged 40 years (EDSS 1; patient B), accessed the emergency department due to respiratory distress and were hospitalized due to interstitial pneumonia confirmed by chest X-ray and subsequent CT scan. They had received the booster dose 139 and 71 days before the infection, respectively, and were both under rituximab treatment. The last dose of rituximab was administered 117 and 180 days before, respectively. Anti-spike IgG antibodies detected after the primary vaccination cycle were below the cut-off in both cases, but information on the titre after the booster dose was not available. Both the patients had fever, cough, and respiratory distress; they received low-flow oxygen therapy, steroids and one non-invasive ventilation; patient A and B were discharged after 20 and 22 days, and the swab became negative after 21 and 34 days, respectively.
3.4. Factors associated with COVID-19
Age at vaccination and time interval (days) between the primary vaccination cycle and the booster dose were independently associated with COVID-19, with HR of 0.95 and 0.98, respectively (p for multivariate analysis 0.013 and 0.015, respectively). No other factors were associated with the infection (Table 3 ).
Table 3.
Factors predictive of COVID-19 infection after the booster dose.
| HR | (95% CI) | P value | |
|---|---|---|---|
| Age, years | 0.96 | (0.92 – 0.99) | 0.030a |
| Sex, female | 0.94 | (0.39 – 2.79) | 0.899 |
| MS form, RR | 1.94 | (0.66 – 5.69) | 0.225 |
| MS duration, years | 0.94 | (1.01 – 1.05) | 0.944 |
| Treatment duration, years | 0.91 | (0.97 – 1.03) | 0.914 |
| Number of previous DMTs | 1.17 | (0.80 – 1.71) | 0.406 |
| Depletive treatment at vaccination | 1.13 | (0.48 – 2.67) | 0.779 |
| Fingolimod or B-cell depleting therapy at vaccination | 1.52 | (0.68 – 3.40) | 0.306 |
| EDSS | 1.11 | (0.91 – 1.36) | 0.290 |
| Time between primary cycle and booster, days | 0.99 | (0.97 – 0.99) | 0.030b |
| Type of vaccine received, Comirnaty | 0.48 | (0.16 – 1.41) | 0.183 |
multivariate analysis: HR 0.95 (95% CI, 0.92 – 0.99); p = 0.013.
multivariate analysis: HR 0.98 (95% CI, 0.97 – 0.99); p = 0.015.
Factors associated with hospitalisation and MS relapse were not explored due to the small number of events observed.
4. Discussion
After the primary vaccination cycle, the administration of a booster dose of anti-SARS-CoV-2 vaccine in population with frailty is recommended by international and national health authorities to reduce the risk of developing severe COVID-19. In Italy, pwMS received with priority the booster dose (either Comirnaty or Spikevax) within a few months from the completion of the primary cycle.
In our cohort, the booster was administered after a median interval period of 6 months, and it was overall well tolerated with an incidence of common adverse events higher than we previously observed in the same cohort after the first two doses (58% vs 33%), but similar to published data (Dreyer-Alster et al., 2022).
The rate of MS reactivation observed in this study within 2 months (2/114, 1.7%) was in line with published cohorts employing Comirnaty only as the booster dose (Dreyer-Alster et al., 2022), but the relatively small sample size of the present study and the lack of a control group represent limitations of the study in providing robust data on the risk of MS relapse.
Vaccine hesitancy in people affected by autoimmune diseases has been raised by previous observations on yellow fever vaccination, leading to an increased risk of MS relapse in 7 patients treated with either interferon or glatiramer acetate (Farez and Correale, 2011). More recently, no association between the same vaccine and MS reactivation was reported in two studies including also patients receiving high-efficacy DMTs (Huttner et al., 2020; Papeix et al., 2021). The proportion of DMT-treated patients was significantly higher in those exposed to this vaccine compared to those not exposed in one study; the difference in time to relapse was not significant between the two groups after adjusting for use of DMTs, but no correction for the DMTs class was performed (Papeix et al., 2021). Incidence of relapses was not different in the pre-exposure compared to the post-exposure period in another study, yet the proportion of untreated patients tended to be higher in those who experienced a relapse compared to those who did not (3/4 vs 10/19, respectively, being natalizumab administered in 8 cases from the latter group), and no details on the treatment status during the pre-exposure period were available (Huttner et al., 2020). Differences in the type and immunogenicity of the vaccine under study, as well as in the rate of treatment with immunosuppressive/immunomodulant drugs and the use of high-efficacy DMTs might account, at least in part, for the differences observed across studies.
Concerning the COVID-19 vaccines, published studies did not observe an increased risk of disease reactivation following the vaccination (Achiron et al., 2021; Di Filippo et al., 2022), yet epidemiological studies are lacking and several phenomena might affect the results, such as short follow-up period, regression towards the mean, and unobserved confounders possibly unbalanced between groups. The scenario is further complicated by the observation that COVID-19 itself may trigger MS reactivation, and that a non-negligible proportion of the vaccinated population will eventually get infected with SARS-CoV-2, strengthening the opportunity to consider the potential additive effects of such events on disease activity.
Over a median follow-up of 6 months after the booster dose, 21% of the patients developed COVID-19, being the event of mild to moderate severity in most cases. This rate is somehow surprisingly high compared to those previously reported (Dreyer-Alster et al., 2022) in vaccinated pwMS, but similar to the incidence of the general population in the same period.
In our cohort there were two hospitalizations, both occurring in patients treated with rituximab, corresponding to 8.3% of the infected cases. This proportion is higher compared to the general population, in which the value attested about 1.5% of the infection in the same period (health, 2022). This could suggest an increased risk of severe COVID-19 and need for hospitalization in patients treated with depletive CD-20 therapies, but our cohort is too small to draw any conclusions and this association has not been not confirmed by a recent study (Dreyer-Alster et al., 2022). Furthermore, even if antibody-mediated immune response to SARS-CoV-2 was reported to be reduced in patients receiving anti-CD20 monoclonal antibodies, an effective cell-mediated immune response may contribute to the protection against COVID-19 in these cases. This was recently suggested by the observation that pwMS treated with anti-CD20 therapies developed a long-lasting anti-SARS-CoV-2 T-cell response after COVID-19, with levels similar to infected persons without MS (Guerrera et al., 2022).
Interestingly, age at vaccination and time interval between the primary vaccination and administration of the booster dose independently predicted SARS-CoV-2 infection, i.e. the younger the patients was at vaccination and the shorter the interval between second and third dose of vaccine, the higher was the risk to develop COVID-19 during follow-up. However, the effect size was small. One possible explanation for these findings could be that unobserved risk factors for COVID-19 could remarkably affect the propensity of each individual to get the infection, such as behavioural and social aspects that are usually not taken into account in retrospective studies. As an example, younger people might be at higher risk of infection due to social behaviour, such as attending crowded places, working activities and living with young children. The administration of the booster dose with priority to patients receiving treatment with depletive DMTs or who had risk factors for severe infection (and therefore who were at higher risk of developing COVID-19) may explain the observed association between shorter interval from primary vaccination to the booster and risk of SARS-CoV-2 infection.
Limitations of the study include the retrospective design, the small sample size and the lack of a control group, which did not allow us to explore whether the incidence of relapses was increased in the post-vaccination period, nor to estimate the effectiveness of the vaccine compared to unvaccinated MS patients. Furthermore, the lack of systematic MRI assessments performed timely before and after the booster dose did not allow us to explore possible subclinical disease activity.
5. Conclusions
The present study confirms an overall good safety profile of the administration of the booster dose in patients with MS; 1.7% of the cases experienced MS reactivation within 4 weeks, but current data do not support an increased risk of MS relapse and further research is needed to answer this question. Three doses of anti-COVID-19 vaccines effectively protected 79% of the patients from infection over a median follow-up of 6 months, and the infection was mild to moderate in most of the cases, without sequelae. Surprisingly, the risk of COVID-19 was associated with both younger age at vaccination and earlier administration of the booster dose. This suggests that unobserved confounders, possibly including behavioural and social factors, play a relevant role in determining the individual propensity to get infected with SARS-CoV-2.
CRediT authorship contribution statement
Andrea Bertozzi: Data curation, Visualization, Writing – original draft. Alice Mariottini: Conceptualization, Data curation, Visualization, Writing – original draft, Writing – review & editing, Supervision. Leonardo Marchi: Investigation, Resources. Maria Di Cristinzi: Investigation, Resources. Riccardo Nistri: Investigation, Resources. Valentina Damato: Investigation, Resources, Writing – review & editing. Claudia Mechi: Investigation, Resources. Alessandro Barilaro: Investigation, Resources. Luca Massacesi: Investigation, Resources. Anna Maria Repice: Conceptualization, Investigation, Resources.
Footnotes
The present work was carried out at the University Hospital of Careggi, Largo Brambilla 3, 50134 Florence, Italy.
Statements and Declarations. The authors received no financial support for the research, authorship, and/or publication of this article. A.B. has no competing interests to declare that are relevant to the content of this article. A.M. reports consultation honoraria from Sanofi and Merck, and non-financial support from , Teva, and Novartis, outside the submitted work. L.M., M. D.C. and R.N. have no competing interests to declare that are relevant to the content of this article. C.M. reports personal fees from Biogen Idec and Novartis, non-financial support from Teva, Merck, Biogen and Novartis. V.D. and A.B. have no competing interests to declare that are relevant to the content of this article. L.M. reports non-financial support from , Novartis, Merck Serono, Genzyme and Teva, outside the submitted work. A.M. R. have no competing interests to declare that are relevant to the content of this article.
Standard Protocols Approval, and Patient Consent. The protocol was approved by the local ethics committee (Tuscan region ethics committee for clinical experimentation; approval number 27569; written informed consent was collected according to local regulations. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, also in the observation of the specific national laws.
References
- Achiron A., Dolev M., Menascu S., Zohar D.N., Dreyer-Alster S., Miron S., Shirbint E., Magalashvili D., Flechter S., Givon U., Guber D., Stern Y., Polliack M., Falb R., Gurevich M. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharbi N.K., Al-Tawfiq J.A., Alwehaibe A., Alenazi M.W., Almasoud A., Algaisi A., Alhumaydhi F.A., Hashem A.M., Bosaeed M., Alsagaby S.A. Persistence of anti-SARS-CoV-2 Spike IgG antibodies following COVID-19 vaccines. Infect Drug Resist. 2022;15:4127–4136. doi: 10.2147/IDR.S362848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., E Markowitz C., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E.T.L., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone F., Lucchini M., Ferraro E., Bianco A., Rossi M., Cicia A., Cortese A., Cruciani A., De Arcangelis V., De Giglio L. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics. 2022;19(1):325–333. doi: 10.1007/s13311-021-01165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M., Cordioli C., Malucchi S., Annovazzi P., Cavalla P., Torri Clerici V., Ragonese P., Nociti V., Radaelli M., Laroni A., Buttari F., Lorefice L., Ferraro D., Gajofatto A., Prosperini L., Fantozzi R., Boffa L., Lanzillo R., Moccia M., Clerico M., De Luca G., Tomassini V., Calabrese M., Borrelli A., Paolicelli D., Maniscalco G.T., Gazzola P., Gallo A., Solaro C., Cocco E., Gasperini C., Tortorella C. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2022;93(4):448–450. doi: 10.1136/jnnp-2021-327200. [DOI] [PubMed] [Google Scholar]
- Dreyer-Alster S., Menascu S., Mandel M., Shirbint E., Magalashvili D., Dolev M., Flechter S., Givon U., Guber D., Stern Y. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J. Neurol. Sci. 2022;434 doi: 10.1016/j.jns.2022.120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R.A., Jawad A.F., Boyer J., Maurer K., McDonald K., Prak E.T.L., Sullivan K.E. Rituximab-treated patients have a poor response to influenza vaccination. J. Clin. Immunol. 2013;33(2):388–396. doi: 10.1007/s10875-012-9813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M., Puopolo M., Morciano C., Spuri M., Alegiani S.S., Filia A., D'Ancona F., Del Manso M., Riccardo F., Tallon M. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: retrospective cohort study. BMJ. 2022:376. doi: 10.1136/bmj-2021-069052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez M.F., Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch. Neurol. 2011;68(10):1267–1271. doi: 10.1001/archneurol.2011.131. [DOI] [PubMed] [Google Scholar]
- Fiolet T., Kherabi Y., MacDonald C.-.J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clinic. Microbiol. Infect. 2021 doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrera G., Mandelli A., Finardi A., Orrico M., D'Orso S., Picozza M., Noviello M., Beretta V., Bonetti B., Calabrese M. Anti-SARS-CoV-2 T-stem cell memory persists in ocrelizumab-treated MS patients. Mult. Scler. J. 2022;28(12):1937–1943. doi: 10.1177/13524585221102158. [DOI] [PubMed] [Google Scholar]
- Huttner, A., Eperon, G., Lascano, A.M., Roth, S., Schwob, J.-.M., Siegrist, C.-.A., Lalive, P.H., 2020. Risk of MS relapse after yellow fever vaccination. A self-controlled case series 7(4), e726. [DOI] [PMC free article] [PubMed]
- Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Mariottini A., Bertozzi A., Marchi L., Di Cristinzi M., Mechi C., Barilaro A., Massacesi L., Repice A.M. Effect of disease-modifying treatments on antibody-mediated response to anti-COVID19 vaccination in people with multiple sclerosis. J. Neurol. 2022:1–8. doi: 10.1007/s00415-022-11003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroianni F., Guida P., Bellanova G., De Nicolò E.V., Righetti G., Formoso M., Celani F. SARS-CoV-2 Antibody response after BNT162b2 mRNA vaccine in healthcare workers: nine-month of follow-up. Vaccine: X. 2022 doi: 10.1016/j.jvacx.2022.100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W.I., Compston A., Edan G., Goodkin D., Hartung H.P., Lublin F.D., McFarland H.F., Paty D.W., Polman C.H., Reingold S.C., Sandberg-Wollheim M., Sibley W., Thompson A., van den Noort S., Weinshenker B.Y., Wolinsky J.S. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Moreno-Torres I., Meca Lallana V., Costa-Frossard L., Oreja-Guevara C., Aguirre C., Alba Suarez E.M., Gomez Moreno M., Borrega Canelo L., Sabin Munoz J., Aladro Y. Risk and outcomes of COVID-19 in patients with multiple sclerosis. Eur. J. Neurol. 2021;28(11):3712–3721. doi: 10.1111/ene.14990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022;23(2):186–193. doi: 10.1038/s41590-021-01122-w. [DOI] [PubMed] [Google Scholar]
- Muñoz-Jurado A., Escribano B.M., Agüera E., Caballero-Villarraso J., Galván A., Túnez I. SARS-CoV-2 infection in multiple sclerosis patients: interaction with treatments, adjuvant therapies, and vaccines against COVID-19. J. Neurol. 2022:1–23. doi: 10.1007/s00415-022-11237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papeix C., Mazoyer J., Maillart E., Bensa C., Dubessy A.L., Goujon C., Launay O., Lebrun-Frénay C., Louapre C., Mrejen S., Pourcher V., Rosenheim M., Stankoff B., Vidal J.S., Lubetzki C. Multiple sclerosis: is there a risk of worsening after yellow fever vaccination? Mult. Scler. 2021;27(14):2280–2283. doi: 10.1177/13524585211006372. [DOI] [PubMed] [Google Scholar]
- Pistor M., Hoepner R., Hoepner A.G., Lin Y., Jung S., Bassetti C.L., Chan A., Salmen A. Multiple Sclerosis immunotherapies and COVID-19 mortality: an analysis of the FDA adverse event reporting system. Ther. Adv. Neurol. Disord. 2022;15 doi: 10.1177/17562864221129383. 17562864221129383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., Lublin F.D., Montalban X., O'Connor P., Sandberg-Wollheim M., Thompson A.J., Waubant E., Weinshenker B., Wolinsky J.S. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrotri M., Krutikov M., Nacer-Laidi H., Azmi B., Palmer T., Giddings R., Fuller C., Irwin-Singer A., Baynton V., Tut G. Duration of vaccine effectiveness against SARS-CoV-2 infection, hospitalisation, and death in residents and staff of long-term care facilities in England (VIVALDI): a prospective cohort study. Lancet Healthy Longev. 2022;3(7):e470–e480. doi: 10.1016/S2666-7568(22)00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormani M.P., Inglese M., Schiavetti I., Carmisciano L., Laroni A., Lapucci C., Da Rin G., Serrati C., Gandoglia I., Tassinari T., Perego G., Brichetto G., Gazzola P., Mannironi A., Stromillo M.L., Cordioli C., Landi D., Clerico M., Signoriello E., Frau J., Ferro M.T., Di Sapio A., Pasquali L., Ulivelli M., Marinelli F., Callari G., Iodice R., Liberatore G., Caleri F., Repice A.M., Cordera S., Battaglia M.A., Salvetti M., Franciotta D., Uccelli A., CovaXi, M.S.s.g.o.b.o.t.I.C.-A.i.M.S. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof S.Y., Slezak J.M., Puzniak L., Hong V., Frankland T.B., Ackerson B.K., Takhar H.S., Ogun O.A., Simmons S.R., Zamparo J.M. Effectiveness of a third dose of BNT162b2 mRNA COVID-19 vaccine in a large US health system: a retrospective cohort study. Lancet Reg. Health-Am. 2022;9 doi: 10.1016/j.lana.2022.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- tracker, C.-v., 2022. COVID19 vaccine tracker. https://covid19.trackvaccines.org/. (Accessed 31 July 2022).
- van Kempen Z.L.E., Wieske L., Stalman E.W., Kummer L.Y.L., van Dam P.J., Volkers A.G., Boekel L., Toorop A.A., Strijbis E.M.M., Tas S.W., Wolbink G.J., Löwenberg M., van Sandt C., Ten Brinke A., Verstegen N.J.M., Steenhuis M., Kuijpers T.W., van Ham S.M., Rispens T., Eftimov F., Killestein J. Longitudinal humoral response after SARS-CoV-2 vaccination in ocrelizumab treated MS patients: to wait and repopulate? Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual de-identified participant data will be shared upon written request.