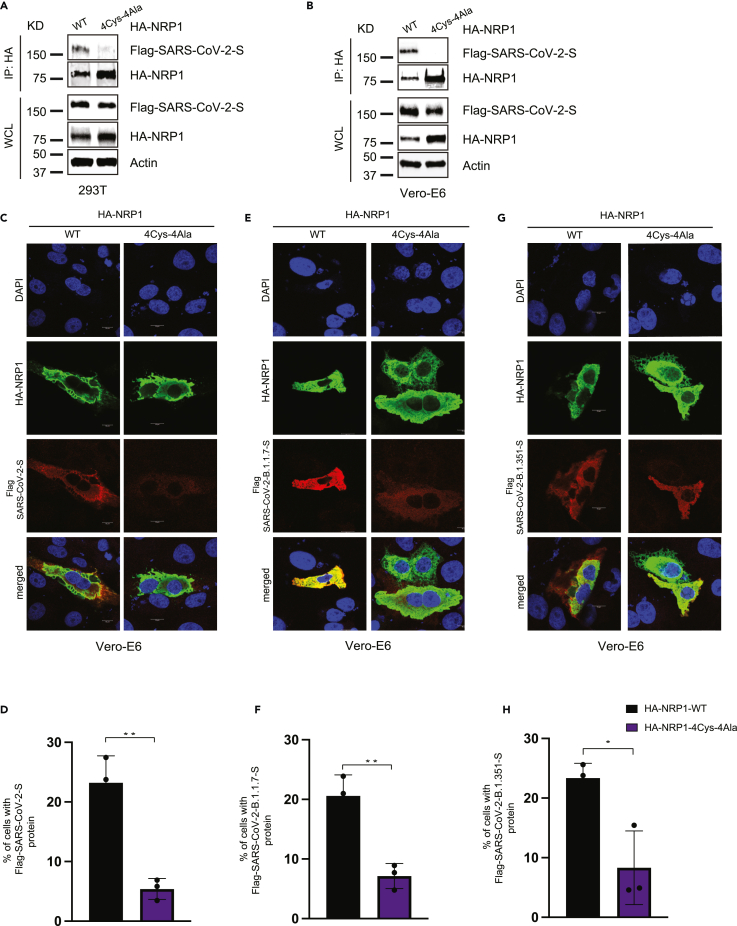
Figure 4.
Mutation in the novel cysteines in NRP1 universally impairs its interaction with different SARS-CoV-2 S protein variants
(A and B) NRP1 PAN domain cysteines play crucial role in SARS-CoV-2 S protein binding. Both 293T and Vero-E6 cells were transfected with the indicated HA-NRP1 (wild type and 4Cys-4Ala mutant) and FLAG-SARS-CoV-2-S constructs. Cells were lysed 30 h post-transfection, and the interaction between HA-NRP1 and FLAG-SARS-CoV-2-S was analyzed.
(C, E, and G) Representative images of colocalization studies between FLAG-tagged different SARS-CoV-2 S protein variants and indicated HA-NRP1 constructs by confocal immunofluorescence microscopy in Vero-E6 cells. The cells were transiently transfected with different FLAG-SARS-CoV-2-S and different constructs of HA-NRP1 as indicated. 30 h post-transfection, cells were fixed and mounted, and protein expression patterns were visualized using a Leica SP8 White Light Laser Confocal System. Scale bars represent 10 μm. The images shown are representative from three independent biological experiments (average 100 cells were observed per experimental condition per replicate).
(D, F, and H) Quantification of the Vero-E6 cells expressing FLAG-SARS-CoV-2-S, FLAG-SARS-CoV-2-B.1.1.7-S, and FLAG-SARS-CoV-2-B.1351-S in the presence of indicated HA-NRP1 constructs. Percentage of cells with FLAG-SARS-CoV-2-S protein was calculated for each of the variant separately and normalized compared to their respective control set (cells with HA-NRP1-WT). Data are represented as mean ± SD, n = 3 (average 100 cells were observed for each condition per experiment), and ∗, p < 0.05; ∗∗, p < 0.005; ∗∗∗, p < 0.0005 were calculated by Student’s t test.