Fig. 2.
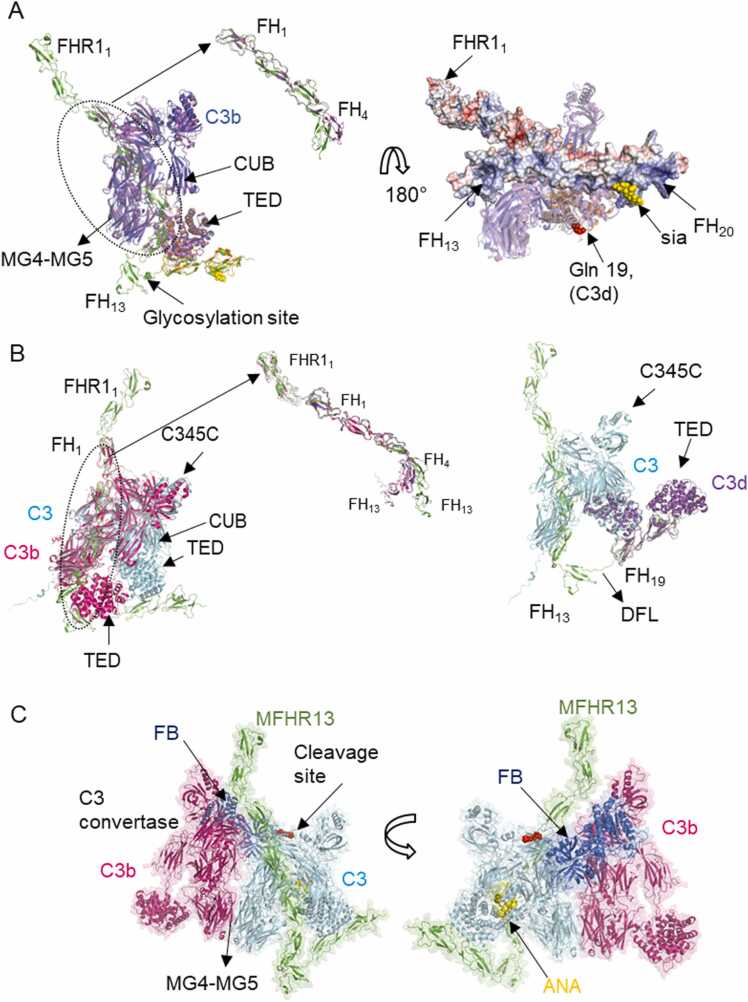
Interaction of MFHR13 with C3 and C3b predicted by AlphaFold-Multimer. A. The model of the complex MFHR13/C3b predicted by AFM agrees with experimental structures of FH fragments in complex with C3b. Superimposition of the model MFHR13 (green) interacting with C3b (blue) with experimental structure of FH1–4/C3b (magenta) (PDB 2wii, RMSD = 1.253 Å), FH19–20/C3d (orange) (PDB 2xqw, RMSD = 0.928 Å) and sialic acid (yellow) (PDB 4ont), (RMSD = 1.253 Å). The thioester-containing domain (TED) and complement C1r/C1s, Uegf, Bmp1 domain (CUB) are indicated by arrows. Zoom in of the superimposition of FH1–4 of the model and the experimental structure is shown. The right panel shows the linkage of C3b (or C3d) to biological surfaces around Gln19 (red spheres) and MFHR13 as a surface with the electrostatic surface potential (electropositive residues in blue and electronegative in red). B. MFHR13 interacts with C3 through FH1–3 and FH19 domains. On the left, MFHR13 and C3 are shown in green and cyan, respectively, and the model is superimposed with C3b in complex with FH1–4 (PDB 2wii, in magenta), where differences in C3 and C3b conformations are observed. RMSD is 2.583 Å for the whole complex superimposed with PDB 2wii, and 1.376 Å for FH1–4, respectively. The right panel shows superimposition of MFHR13/C3 model (green/cyan) with experimental structure FH19–20/C3d (PDB 2qxw, in purple). RMSD is 0.601 Å for C3 TED domain and C3d. The disordered flexible linker (DFL) is shown. C. C3 convertase (C3bBb) in complex with C3/MFHR13 (enzyme-substrate complex). Superimposition of C3 in MFHR13/C3 model with MG4-MG5 domains of C3b molecule in C3 convertase (PDB 2win, C3b in magenta, Bb fragment in dark blue). MFHR13 is shown in green and C3 in light blue with the anaphylatoxin domain (ANA) in yellow and the cleavage site as red spheres. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)