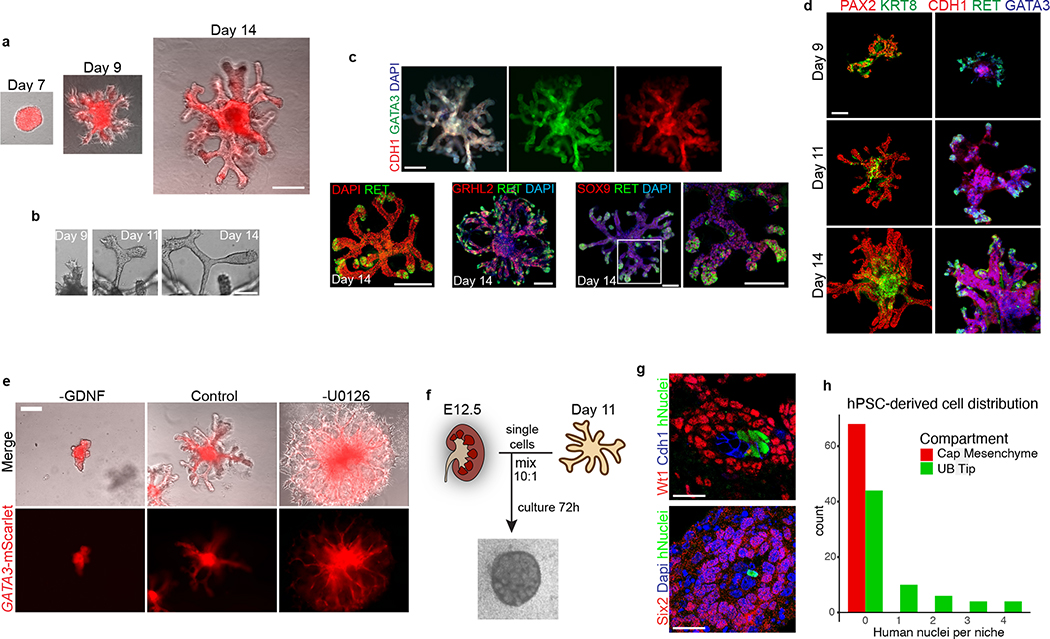
Figure 2. Three-dimensional development of branching UB organoids.
a, Between days 7–14, the nephric duct spheres underwent extensive remodeling and growth into UB organoids, which grew in a pattern of elongating stalks and branching tips similar to the fetal UB. b, Bifurcative branching was frequently observed over this time period. c, Molecular analysis at day 14 reiterated a complex branched architecture and the entire epithelium exhibited expression of GATA3, GRHL2, SOX9, and CDH1. The progenitor marker and GDNF co-receptor RET was exclusively expressed in in the distal tip domains. d, Wholemount IF staining at days 9, 11, and 14 revealed uniform expression of PAX2 with progressive segregation of the tip (RET) and stalk (KRT8) domains. e, In the absence of exogenous GDNF, the nephric duct spheres largely failed to grow and branch. Conversely, removal of the MEK inhibitor U0126 from the culture medium led to a more disorganized branched plexus of fine epithelial cords with terminal filopodia rather than rounded UB-like tips. f, To functionally assess the competence of UB organoid cells, we mixed them with dissociated murine kidneys isolated at embryonic day (E) 12.5, pelleted the cells into chimeric aggregates, and cultured on filter disks for 72 hours. g, IF staining showed the incorporation of induced UB cells (indicated by a human-specific antibody) into the Cdh1-expressing UB tip domains but not in the Six2+Wt1+ nephron progenitor compartment. h, Histogram depicting the frequency at which we observed a human cell or cluster of cells in the examined progenitor niches. Scale bars, 200 μm (a and c-e), 100 μm (b) and 20 μm (g).