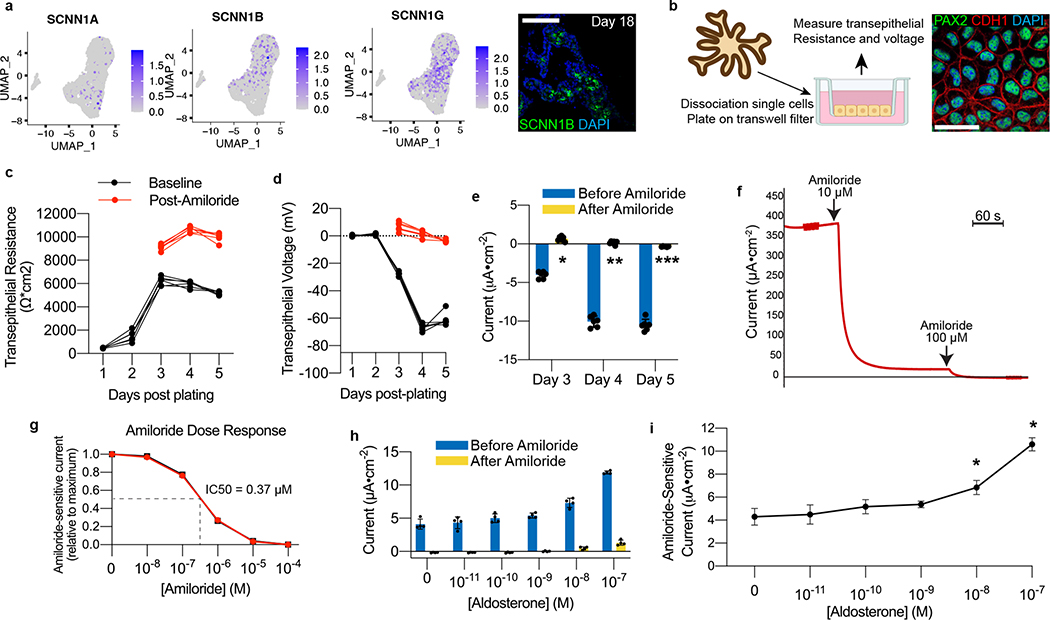
Figure 4. hPSC-derived model for interrogating ENaC-mediated sodium transport.
a, Expression of the ENaC subunits SCNN1B and SCNN1G was abundant in scRNA-seq data, and we detected SCNN1B at the protein level in day 18 organoids. b, To explore functional capabilities, organoids at day 18 were dissociated and plated onto two-dimensional transwell filters for electrophysiological interrogation. This led to confluent epithelia that maintained expression of collecting duct markers PAX2 and CDH1. c-d, As the cells became confluent, there was at first a steep increase and then plateau in the transepithelial resistance, which was then followed by the emergence of a transepithelial voltage. The voltage was entirely suppressible within three minutes of addition of the ENaC antagonist amiloride (10 μM), which also raised the resistance across the epithelium. e, The resulting current generated was calculated using Ohm’s Law, and it was completely ablated immediately following addition of amiloride, confirming the conductance was ENaC-dependent. *p=5.3×10−9, **p=5.7×10−8, ***p=4.5×10−6; two-tailed student’s t-test comparing results before and after amiloride; n=6 biological replicates, data representative of 3 independent experiments. f, Under closed-circuit conditions with voltage clamping in Ussing chamber, the current produced by the epithelium was similarly sensitive to amiloride, and it was completely abolished upon increasing the concentration to 100 μM. g, Amiloride induced a dose-dependent inhibition of transepithelial current in Ussing chamber, with representative dose-response curves shown from two independent wells. h-i, Addition of aldosterone for 24 hours led to a dose-dependent increase in transepithelial current measured under closed circuit conditions, and up to a maximum of approximately 2.5-fold increase in amiloride-sensitive current. *p<0.005; two-tailed student’s t-test compared to no aldosterone; n=4 independent biological replicates. Scale bars, 100 μm (a) and 20 μm (b). Column and error bars represent mean and standard deviation, respectively.