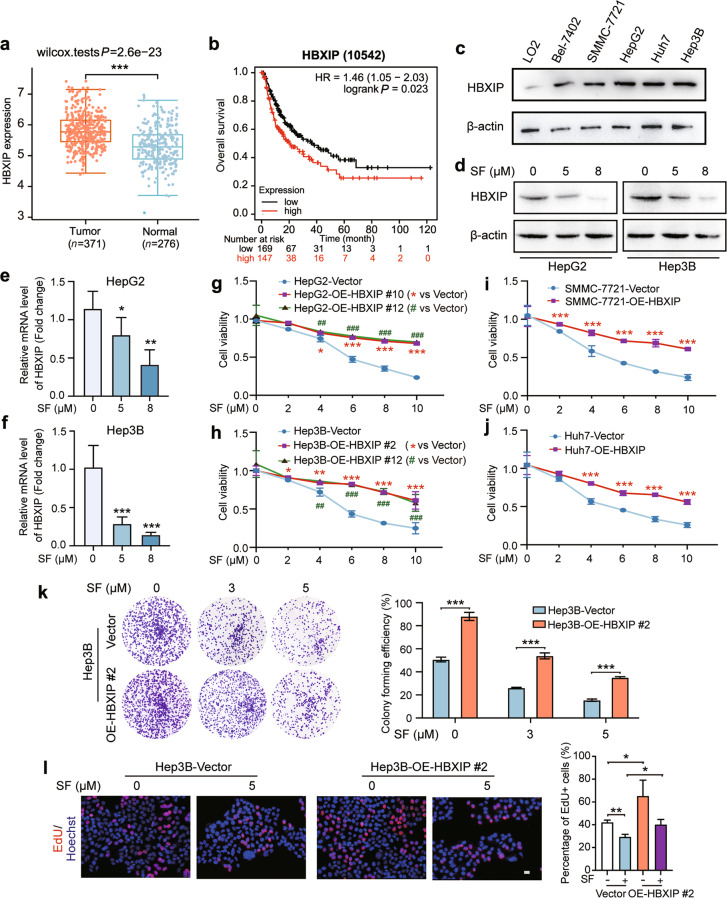
Fig. 1. Oncoprotein HBXIP is decreased by treatment with sorafenib in HCC.
a The HBXIP expression level in 371 HCC patients’ tumor tissues and 276 normal tissues from the TCGA dataset. b Kaplan–Meier plots of the overall survival of 316 HCC patients grouped by HBXIP mRNA levels from the TCGA dataset. c Western blotting (WB) analysis was performed to analyze the expression of HBXIP in LO2, Bel-7402, SMMC-7721, HepG2, Huh7, and Hep3B cells. d WB analysis was used to test the expression of HBXIP in HepG2 and Hep3B cells dose-dependently treated with DMSO or sorafenib (SF) for 48 h. e, f Fold change in mRNA levels of HBXIP determined by RT-qPCR assays in HepG2 and Hep3B cells dose-dependently treated with DMSO or sorafenib for 48 h. g, h MTT assay was utilized to examine cell viability in the indicated stable cells under the treatment of sorafenib. *P < 0.05, ***P < 0.01 or ##P < 0.01, ###P < 0.001 represented the P value of the cell viability in different OE-HBXIP group compared to the Vector group. i, j MTT assay was applied to evaluate the cell viability in SMMC-7721 and Huh7 cells transfected with pcDNA-Vector or pcDNA-HBXIP and treated with the indicated concentrations of sorafenib. ***P < 0.001. k A colony photograph of Hep3B and stable Hep3B-OE-HBXIP #2 cells dose-dependently treated with the indicated concentrations of sorafenib. l EdU incorporation assay in the indicated stable cells treated with DMSO or 5 μM sorafenib for 48 h. EdU positive cells were quantified by percentage of EdU+ (Red) /Hoechst+ (Blue). Scale bar = 20 μm. Columns with error bars symbolize the average of three independent replicates ± SD. Three experiments with consistent results tendency were analyzed by one-way ANOVA (e, f, k and l) or two-way ANOVA (g–j). ***P < 0.001, **P < 0.01, *P < 0.05.