Table 1.
Optimizing the three-component reaction of 4-chlorobenzaldehyde (2a), malononitrile (3), and dimedone (4a) under various conditions.
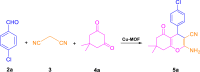 .
.
| Entry | Catalyst loading | Solvent | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|
| 1 | 0.04 | THF | 75 | 360 | 68 |
| 2 | 0.04 | MeCN | 75 | 300 | 85 |
| 3 | 0.04 | EtOH | 75 | 20 | 90 |
| 4 | 0.04 | EtOH | 50 | 30 | 85 |
| 5 | 0.04 | EtOH | r.t | 45 | 80 |
| 6 | – | Solvent-free | r.t | 80 | 88 |
| 7 | 0.02 | Solvent-free | r.t | 26 | 90 |
| 8 | 0.04 | Solvent-free | r.t | 5 | 96 |
| 9 | 0.04 | Solvent-free | r.t | 20 | 78 |
a Conditions of reaction: 4-chlorobenzaldehyde (2a, 1.0 mmol), malononitrile (3, 1.0 mmol), dimedone (4a, 1.0 mmol), grinding, ambient temperature.
Yield refers to isolated products.
