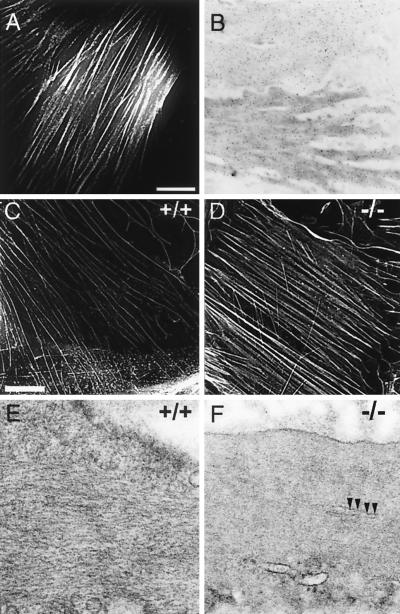
FIG. 5.
Cytoskeletal organization of wild-type and SM22α-deficient SMCs. (A) Cellular localization of Ds-Red-tagged SM22α in A7r5 SMCs. 3-D fluorescence images of A7r5 cells that stably express Ds-RED epitope-tagged SM22α were acquired using a Delta Vision micrscopy system, and image restoration and volume projections were computed as described in Materials and Methods. SM22α protein (white lines) localized to actin filament bundles. (B) Subcellular distribution of SM22α in the intact mouse aorta. Ultrathin sections of mouse aorta were incubated with affinity-purified anti-SM22α polyclonal antiserum. The sections were then incubated with gold-conjugated anti-rabbit IgG and stained with uranyl acetate. The anti-SM22α antibody (black dots) hybridized to longitudinally oriented actin filaments located throughout the cytoplasm and at the cell periphery. (C and D) 3-D fluorescence images of primary mouse aortic SMCs isolated from a wild-type (C) and SM22α-deficient (D) mouse stained with rhodamine-labeled phalloidin. Actin filament bundles (white lines) form well-organized rib-like arrays in the SM22α-deficient mouse (−/−, D) and its control littermate (+/+, C). No significant differences in fluorescence intensity in staining were detected. Bar, 1 μm. (E and F) Ultrastructural comparison of SMCs in the intact bladder of wild-type (E) and SM22α-deficient (F) mice. The bladder was fixed, embedded, sectioned, and stained, and images were generated with a Philips CM-100 electron microscope as described in Materials and Methods. Compared to the SMCs in the wild-type bladder which demonstrate obvious well-spaced, longitudinally oriented actin filaments (+/+, F), the cytoplasm of SM22α-deficient SMCs appears to be homogeneous, reflecting differences in spacing of the actin filaments. This resulted in more-condensed-appearing cytoplasmic bundles (arrows). Magnification, ×104,125.