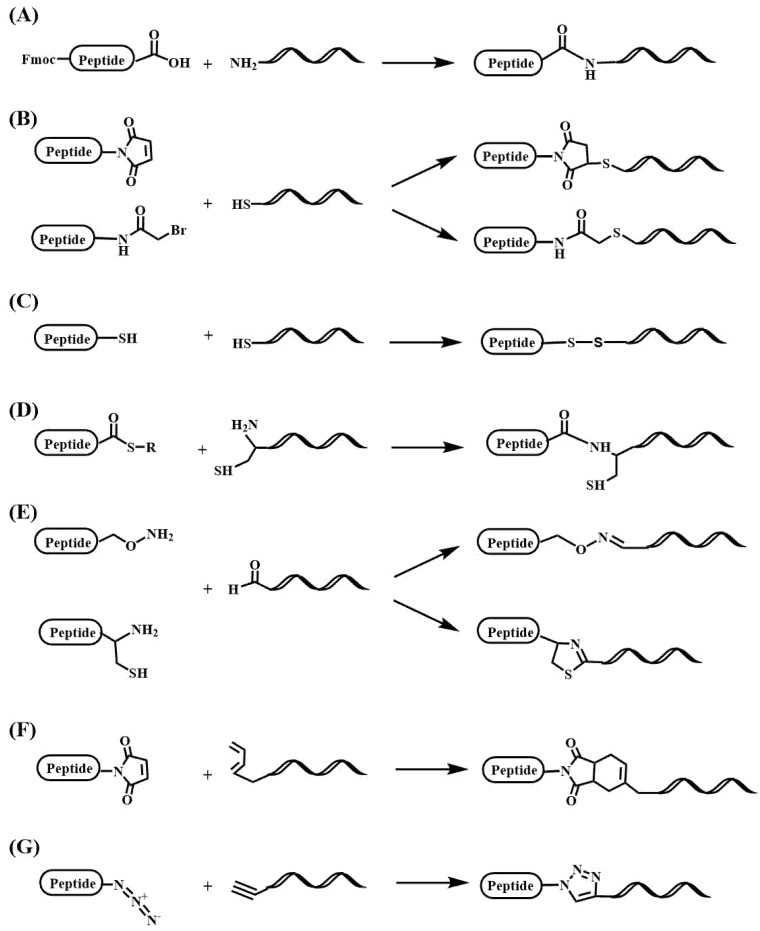
Figure 5.
Postsynthetic reactions for the synthesis of oligonucleotide-peptide conjugates. (A) Using protected peptide segments for fragment condensation [175], (B) thiol-maleimido and thiol-bromoacetamido reactions [176], (C) disulfide formation [177,178,179], (D) synthesis by native ligation [180], (E) oximes or thiozolidine reactions [181,182,183], (F) conjugation by Diels-Alder reaction [184,185], (G) conjugation reactions catalyzed by copper to produce alkyne-azide cyclo additions [186,187,188].