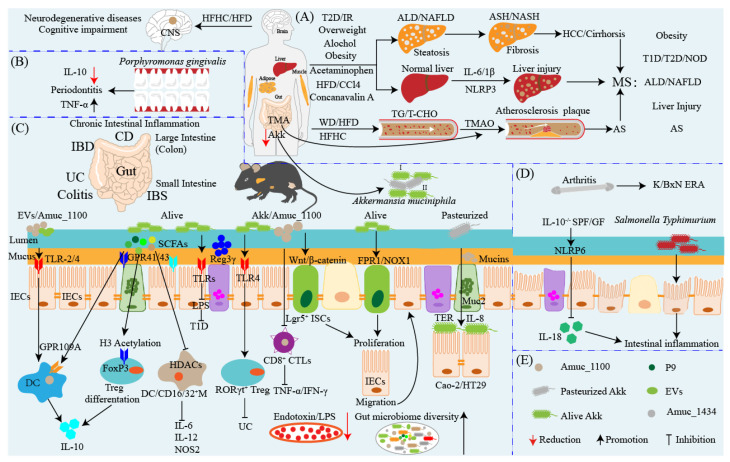
Figure 2.
The duality and challenge of Akkermansia muciniphila in metabolic syndrome and inflammation therapy. Metabolic syndromes include ALD, NAFLD, NASH, neurodegenerative diseases induced by diets such as HFD/HFHC, and atherosclerosis, and have become one of the major challenges of diseases affecting global public health. Akk as the next promising candidate probiotic shows outstanding antimetabolic syndrome potential. (A) Long-term HFD/HFHC diets usually result in neurodegenerative diseases such as cognitive impairment, impaired spatial working memory and novel object recognition, and damaged brain metabolism in early life, while supplementation with Akk can prevent it. T2D, overweightness, obesity, and long-term alcohol consumption generally accompanied by and/or leading to NAFLD/ALD, which include simple steatosis, fibrosis, and cirrhosis, all of which can deteriorate towards ASH/NASH and ultimately deteriorate to HCC. Dietary supplement of Akk ameliorates NAFLD/ALD via regulating lipid and glucose metabolism and intestinal microbiota and eventually improves body metabolic health. Furthermore, HFD usually induces chronic liver injury and inflammatory response, whereas supplementation with Akk saves it by regulating lipid metabolism and inhibiting inflammation. AS is the main contributor to cardiovascular mortality, which is mainly induced by TG/T-CHO, excessive accumulation in WD/HFD/HFHC diets and results in narrowed or blocked blood vessels that drive the cardiovascular events that occur. TMA, one of the metabolites of choline, is metabolized by the gut microbiota to produce TMAO that is proatherogenic and accelerates PROGRESSION, whereas Akk regulates lipid metabolism and inflammatory response to inhibit AS. (B,C) The anti-inflammation and proinflammatory function of Akk in the gut and other organs. (B) Akk ameliorates porphyromonas-gingivalis-induced periodontitis by inhibiting bone destruction and TNF-α proinflammatory cytokine secretion and upregulating IL-10 production. (C) Akk improves chronic and acute intestinal inflammation including IBD, colitis, IBS, and pathogenic or fungal infection by multiple signaling pathways. For instance, EVs and/or Amuc_1100 activate TLR2/4 to stimulate DCs and secrete anti-inflammatory cytokine IL-10 to repress inflammation. Akk also produces butyrate, which can activate the G protein-coupled receptor 109A (GPR109A) of DCs to promote IL-10 production. Butyrate also directly suppresses the HDACs of DCs/macrophages to inhibit the production of IL-6, IL-22, and NOS2. Propionate, one of the SCFAs derived from Akk, activates GPR43 of Tregs to promote Tregs differentiation to secret IL-10. Akk represses CD8+ CTLs to inhibit the production of TNF-α and IFN-γ as well as activates TLRs to inhibit LPS-induced type 1 diabetes (T1D). Akk abundance is decreased and the TLR4 expressing level is upregulated in patients with UC and TLR4 knockout exacerbates intestinal inflammation and is accompanied by the reduction in Akk, whereas supplementation of Akk ameliorates colitis by upregulating RORγt+ Treg cell-mediated immune responses. The colonization of Akk is triggered by the interaction of TLR4 and Amuc_1100. Akk not only represses the production and translocation of endotoxin/LPS and recovers the diversity of the microbiota, but also promotes the secretion of antimicrobial peptides and mucins and TJ expression to enhance intestinal barrier integrity. In addition, Akk and Amuc_1100 promote intestinal stem cell proliferation mediated by Wnt/β-catenin and/or FPR1/NOX1 signaling pathways to accelerate intestinal epithelial renewal and protect the gut barrier. Akk enhances transepithelial resistance and inhibits IL-8 production to enhance intestinal epithelial integrity. In addition to intestinal inflammation, gut endotoxin/LPS translocates into the circulatory system and reaches the liver, resulting in liver injury. LPS, acetaminophen, CCl4, and Concanavalin A all can induce acute liver injury, which triggers a severe inflammatory response mediated by NLRP3 inflammasome activation to produce more proinflammatory cytokines IL-6 and IL-1β. (D) Akk acts as a pathobiont to promote colitis and exacerbate inflammation progress in some specific conditions. For example, Akk is partly increased in children with enthesitis-related arthritis (ERA), and the administration of the fecal mass of the KRN/B6 × NOD (K/BxN) model of RA mice treated with Akk to human patients with ERA slightly increases ankle swelling and arthritis. Akk exacerbates the inflammatory response caused by murine Salmonella enterica Typhimurium and DSS. In both specific pathogen-free and germ-free IL10-/-mice, the colonization of Akk mediated by NLRP6 is sufficient for promoting intestinal inflammation by inhibiting IL-18 production modulating the abundance of Akk. (E) The forms of Akk and its outer membrane components. Abbreviations: ALD: alcoholic liver disease; ASH: alcoholic steatohepatitis; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; HCC: hepatocellular carcinoma; WD: Western diet; HFD: high-fat diet; HFHC: high-fat high-cholesterol; TLRs: Toll-like receptors; LPS: lipopolysaccharide; TG: triglyceride; T-CHO: total cholesterol; AS: atherosclerosis; TMA: trimethylamine; TMAO: trimethylamine-N-oxide; T2D: type 2 diabetes; T1D: type 2 diabetes; NOD: non-obese diabetes; IBD: inflammatory bowel disease; CD: Crohn’s disease; UC: ulcerative colitis; IBS: irritated bowel syndrome; CD8+ CTLs: CD8+ cytotoxic T lymphocytes; CCl4: carbon tetrachloride; SCFAs: short-chain fatty acids; ERA: enthesitis-related arthritis; K/BxN: KRN/B6xNOD mice; M: macrophages; Tregs: regulatory T cells; DCs: dendritic cells; HDACs: histone deacetylases; TJ: tight junction; MS: metabolic syndromes.