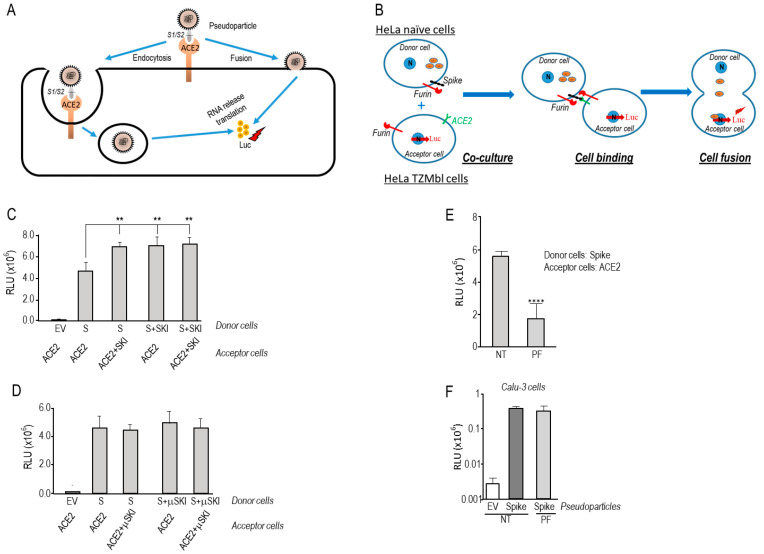
Figure 1.
SKI-1 activity is required for cell-to-cell fusion in HeLa cells but not for pseudoparticle entry. (A) Schematic representation of pseudotyped particles’ entry into Calu-3 cells. Upon incubation of cells with nanoluciferase-expressing HIV particles pseudotyped with SARS-CoV-2 S WT, pseudoparticles can either enter the cell by endocytosis or fusion upon Spike priming and activation. After RNA release and nanoluciferase expression, the extent of fusion is quantified by measuring nanoluciferase activity. (B) Cell-to-cell fusion between donor cells (HeLa) expressing the SARS-CoV-2 spike protein along with the HIV trans-activator Tat, and acceptor cells (TZM-bl) that express ACE2. Upon fusion, Tat is transferred from donor to acceptor cells, thereby inducing luciferase expression. (C,D) Hela cells transiently transfected with an empty vector (EV) or expressing SARS-CoV-2 spike (S) (donor cells), were co-cultured for 18 h with TZM-bl HeLa cells expressing ACE2 receptor (acceptor cells). The values shown are the (C) WT SKI-1 or (D) its active site mutant (μSKI-1, H249A), which were expressed in acceptor and/or donor cells. (E) Donor HeLa cells expressing WT-S were co-cultured with acceptor TZM-bl cells expressing ACE2 in absence or presence of 10 μM of SKI-1 Inhibitor (PF429242). (F) Calu-3 cells were inoculated with nanoluciferase-expressing HIV particles pseudotyped with empty vector (EV) or SARS-CoV-2 wild-type spike (WT), in the absence or the presence of 10 μM of SKI-1 Inhibitor (PF429242). The cell-to-cell fusion luciferase assay used throughout is detailed in the Material and Methods Section 2.4. The relative light units (RLU) were obtained using a Promega GloMax plate reader luminescence detection system. Representative data from at least three independent experiments are shown. p values (**, p < 0.01; ****, p < 0.0001) were evaluated by a Student’s t-test.