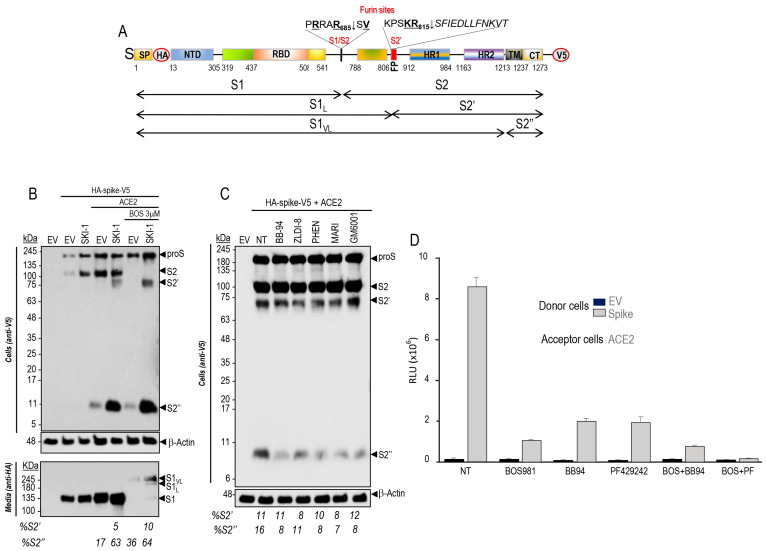
Figure 2.
SKI-1 increases the cleavage at S2′ and sheds the spike glycoprotein. (A) Schematic representation of the primary structure of preproS, including its domains, the predicted furin-like S1/S2 site generating the S1- and S2-subunits, and the S2′ site preceding the fusion peptide (FP). The signal peptide (SP), N-terminal domain (NTD), receptor binding domain (RBD) to ACE2, the two heptad repeats HR1 and HR2, the transmembrane domain (TM), the cytosolic tail (CT) and the C-terminal V5-tag are indicated. (B) Western blot analyses of cell extracts and media from HeLa cells following co-transfection of cDNAs coding for doubly tagged WT proS with empty vector (EV) and/or that coding for ACE2 and SKI-1, and treatment with vehicle control (DMSO) or with 3 μM of the furin inhibitor BOS-981. The cell extracts (upper panel) and the media (lower panel) were analyzed using anti-V5 and anti-HA mAbs, respectively. The sizes and schematics of the generated fragments are indicated in (A), and the migration positions in (B). (C) Hela cells transiently transfected with a cDNA encoding an empty vector (EV) or with one expressing the V5-tagged spike (S) glycoprotein were treated, 24 h post- transfection, with vehicle DMSO (NT) or with 1 μM of the indicated metalloproteinase inhibitors. The cell extracts were analyzed by Western blotting using an mAb-V5. The percentage of shed S2″ is indicated. (D) Naive Hela cells (donor) were transfected with vectors expressing either no protein (EV) or WT-spike (S). TZM-bl HeLa cells (acceptor) were transfected with a vector expressing ACE2. After 48 h, donor and acceptor cells were co-cultured for 18 h in the absence (NT) or presence of the furin inhibitor (BOS-981, 3 μM) and/or metalloproteinase inhibitor (BB, 1 μM) and SKI-1 inhibitor (PF429242, 10 μM). The extent of fusion is represented as relative luminescence units (RLU).