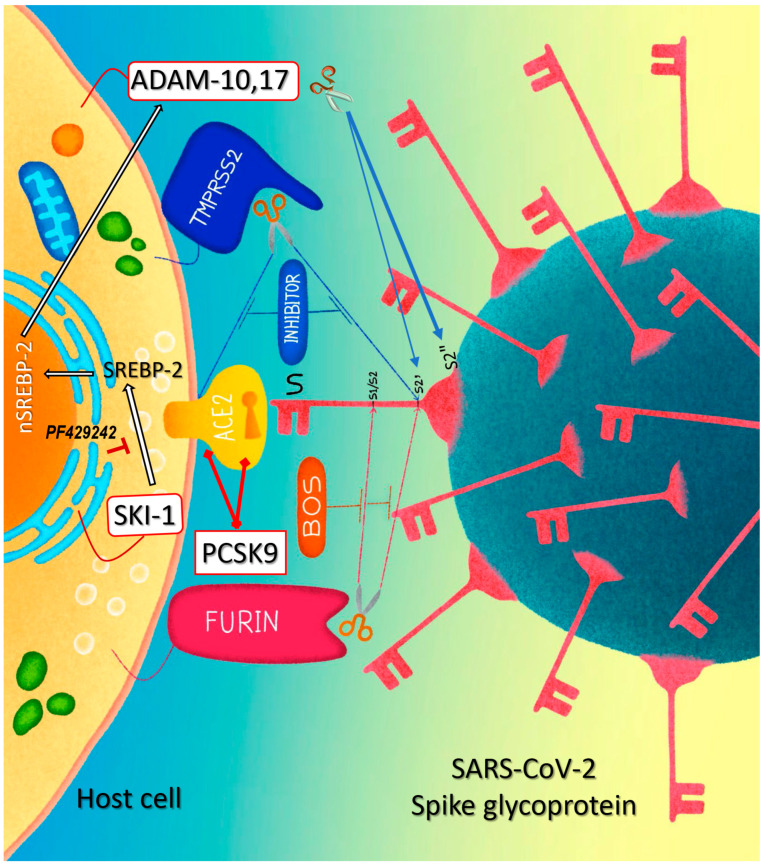
Figure 9.
Graphical representation of a working model of SKI-1 and PCSK9 effects on cell-to-cell fusion. At low level of sterols in the cell, SREBP-2 moves from the ER to Golgi where it is cleaved by SKI-1 and then activated by S2P [29], the N-terminal domain of SREBP-2 (nSREBP-2) is then translocated to the nucleus and triggers the transcription of genes that are involved in cholesterol synthesis [102]. In turn, cholesterol increases the activity of metalloproteinases at cell surface, notably ADAM10 and ADAM17 [24]. The activated metalloproteinases enhance the cleavage of the S-glycoprotein at S2′ and increase cell-to-cell fusion [21]; they also shed the protein by cleaving it at S2″ close to the TMD (this work). The cleavage at S2′ site can also be achieved by furin and TMPRSS2, and the latter sheds ACE2 as previously shown [9]. The combination of SKI-1 and/or metalloproteinase inhibitors with a furin-like inhibitor can be a potent tool to control cell-to-cell fusion and viral entry. Extracellular PCSK9 enhances the degradation of ACE2 by a mechanism requiring only the prodomain/catalytic subunit of mature PCSK9 that likely bind ACE2, thereby reducing cell surface levels of ACE2, cell-to-cell fusion, and possibly SARS-CoV-2 infection.