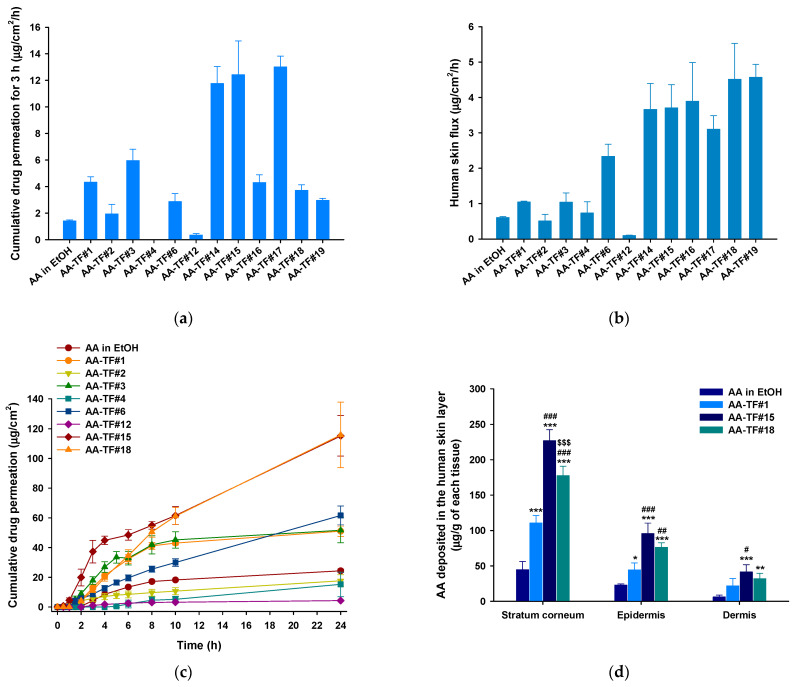
Figure 2.
In vitro full-thickness human skin permeability of atraric acid (AA) in ethanol (EtOH) and topical formulations (AA–TFs): (a) Cumulative drug infiltration in human skin during the initial 3 h after treatment; (b) Skin flux with 1% AA in EtOH or AA–TFs over 24 h; (c) Time-course curve of cumulative AA permeation infiltration of 1% AA in EtOH or AA–TFs for 24 h; (d) AA deposited in the human stratum corneum, epidermis, and dermis using 1% AA in EtOH, AA–TF#1, AA–TF#15, and AA–TF#18 24 h after loading. * p < 0.05, ** p < 0.01, and *** p < 0.001 compared to AA in EtOH; # p < 0.05, ##p < 0.01, ### p < 0.001 compared to AA–TF#1; $$$ p < 0.001 compared to AA–TF#15. Values are presented as means ± SDs (n = 4 for each group).