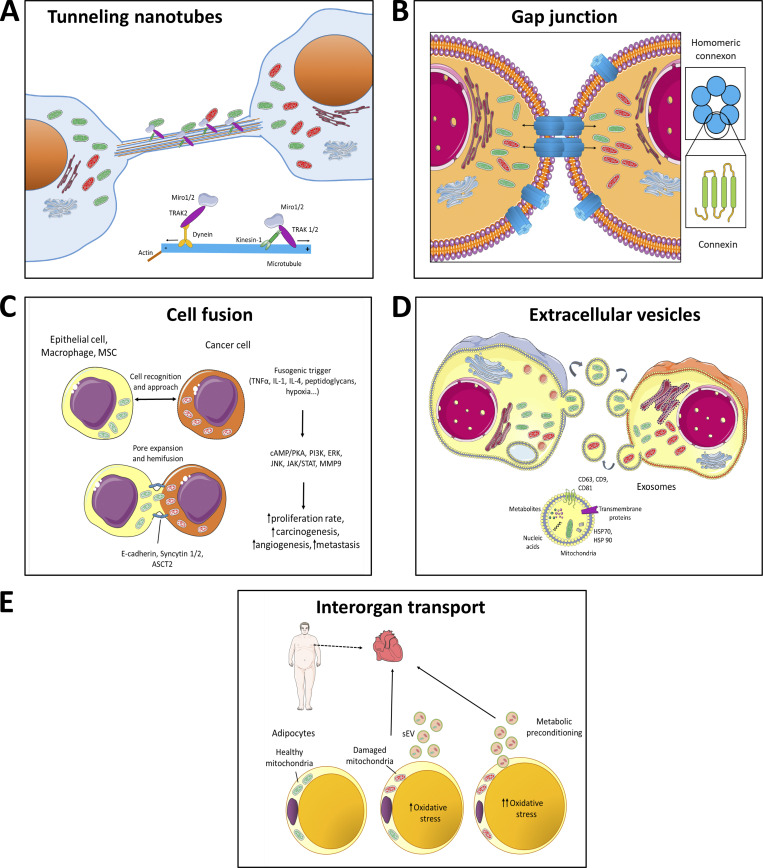
Figure 2.
Models of horizontal transfer of mitochondria. (A) Horizontal transfer of mitochondria by TNTs. TNTs are in general formed by F-actin filaments. In case of intercellular transfer of mitochondria, TNTs also contain microtubules and are thicker, thus able to transport bulkier structures. Transport of mitochondria along these cytoskeletal elements is propelled by dynein and kinesin motor complexes consisting of several adaptor proteins, such as Miro1 or Miro2, that are integrated in the outer mitochondrial membrane and facilitate mitochondrial transport not only along microtubules but also along actin filaments (together with Myo19). Formation of TNTs starts either as an actin-driven protrusion of the cell membranes of the two cells involved or as the dislodgement of two previously attached cells that during the partition from each other form the TNT. (B) Horizontal transfer of mitochondria by gap junctions. Gap junctions were shown to involve connexin structures, i.e., protein complexes consisting of six subunits of connexin proteins, such as Cx43. Two juxtapositioned connexon channels form pores connecting two neighboring cells, allowing for bidirectional transport of ions, signaling molecules or whole mitochondria. (C) Horizontal transfer of mitochondria by cell fusion. Cell fusion is a process that is relatively common in cancer progression and comprises several steps. At first two cells are recognized via the so-called fusogenic trigger that could involve different signaling molecules depending on the cell type (TNFα, IL-1, IL-4, and others) or specific conditions (e.g., hypoxia). The cells then approach each other and, using several cell–cell adhesion molecules, such as E-cadherin, syncytin-1 and -2, or ASCT2, which form pore expansions, “fusion” of the two neighboring cells occurs. This yields a cell that shares mitochondria from the two original cells. During this process, several signaling pathways are triggered that result in higher tumorigenesis and increased metastatic potential of the cancer cells. (D) Horizontal transfer of mitochondria by ECVs. Transfer of mitochondria via ECVs involves small double membrane structures that are formed by blebbing of plasma membrane. In contrast, exosomes are of endosomal origin and can transport various cargo including signaling molecules (different metabolites), trans-membrane proteins, nucleic acids, amino acids, and organelle fragments. (E) Inter-organ transport of mitochondria. This mode of mitochondrial horizontal transfer has been recently shown for the organelles moving from adipocytes to the heart tissue. In this particular case, damaged mitochondria from energetically stressed adipocytes of obese patients are transferred via small extracellular vesicles (sEV) to the blood circulation and are taken up by CMs of the heart tissue, triggering small ROS burst. This process results in compensatory antioxidant signaling in the heart muscle, causing metabolic pre-conditioning.