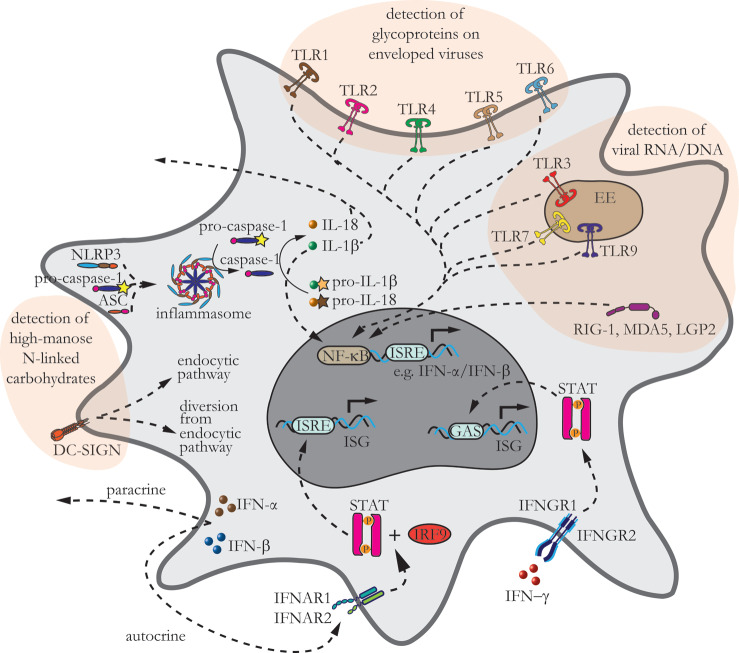
Figure 2.
Astrocytes express several pattern recognition receptors (PRRs), which detect viral infection. The schematic depicts an astrocyte with a variety of PRRs engaged in triggering signaling cascades of the innate immune response. TLR1, TLR2, and TLRs 4-6, are located at the plasma membrane and detect glycoproteins on the viral envelope, whereas TLR3, TLR7, and TLR9 are located at the membrane of early endosomes. TLR3 detects double-stranded intermediate RNA (dsRNA), TLR7 detects single-stranded RNA (ssRNA), and TLR9 detects double-stranded DNA (dsDNA). RLRs are localized in the cytosol and sense viral RNA and DNA; they comprise RIG-I, melanoma differentiation-associated protein 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2). Nucleotide oligomerization domain (NOD)-like receptors (NLRs) are another group of PRRs in the cytoplasm of astrocytes. NLRP3 contains pyrin N-terminal effector-binding domain (NLRPs), which together with pro-caspase-1 and ASC, make up the inflammasome; here, pro-inflammatory caspase-1 is converted to active caspase-1, which processes inactive interleukin (IL) precursors pro-IL-1β and pro-IL-18 to their active forms IL-1β and IL-18. IL-1β and IL-18 are either secreted from cells or activate nuclear factor-kappa B (NF-κB). CLRs are expressed at the cell surface, where they detect high-mannose N-linked carbohydrates on endogenous molecules and pathogens. Dendritic cell (DC)-specific ICAM-3 grabbing non-integrin (DC-SIGN) internalizes viruses that can either enter endocytotic pathway, ultimately resulting in viral degradation and antigen presentation, or can escape endocytic pathway. The CLR-related viral cycle in astrocytes has been poorly addressed thus far. PRR-triggered activation and translocation of IFN-regulatory factors (IRFs) and NF-κB initiate the production of inflammatory cytokines and type I interferons (IFNs), IFNα/β. IFNα/β act in paracrine and autocrine ways via interferon receptors (IFNARs) through which they initiate signaling cascades, leading to the transcription of interferon-stimulated genes (ISGs) acting through IFN-stimulated regulatory elements. IFN-γ secreted by antigen-presenting cells further assists IFNα/β in clearing virus from the tissue. IFN-γ acts through interferon-gamma receptor (IFNGR) protein complex IFNGR1 and IFNGR2, initiating a signaling cascade on gamma interferon activation site (GAS) elements in the DNA, inducing cytokine transcription.