Abstract
Background
Recent studies indicated that the prognosis of patients with gastrointestinal tumors is frequently influenced by its complications, notably myocardial injury. The main object is to investigate the occurrence and risk factors of myocardial injury in patients with gastrointestinal tumor.
Methods
1126 patients who received gastrointestinal tumor related surgery from May 2018 to June 2020 in the Sixth Affiliated Hospital of Sun Yat-sen University were retrospectively collected and divided into the non-myocardial injury group and the myocardial injury group (high-sensitive cardiac troponin I (hs-cTnI) ≥ 0.028 ng/ml). The occurrence and risk factors of myocardial injury in patients with gastrointestinal tumor are analyzed. The influence of myocardial injury on the ICU detention time in gastrointestinal tumor patients is also studied.
Results
In total, 78 (6.93%) patients developed myocardial injuries. Compared with patients in the non-myocardial injury group, patients in the myocardial injury group have a higher prevalence of cardiovascular risk factors (including advanced age and higher smoking ratio), a higher prevalence of comorbidities (such as previous coronary artery disease, hypertension, atrium fibrillation and diabetes), and a higher rate of premedication (such as anticoagulation, β-blocker, Angiotensin-converting enzyme inhibitor/Angiotensin II receptor blocker, and diuretic) (all with P-value < 0.05). In addition, patients in the myocardial injury group also presented with a higher revised cardiac risk index (Lee index), higher neutrophil granulocyte ratio, lower hemoglobin, and higher likelihood of impaired cardiac structure and function (all with P-value < 0.05). There was a trend of statistical significance in the ICU detention time between the myocardial injury group and the non-myocardial injury group (1[1,3] vs. 2[1,10], P = 0.064). In this study, there were 7 patients presented with clinical symptoms in the myocardial injury group (chest discomfort in 4 cases, non-compressive precordial chest pain in 1 case, dyspnea in 2 cases). In the multivariate analysis, advanced age, increased Lee index score, increased neutrophil granulocyte ratio, decreased left ventricular ejection fraction (LVEF), increased interventricular septum were independent risk factors for myocardial injury.
Conclusion
In conclusion, advanced age, increased Lee index, increased neutrophil granulocyte ratio, decreased left ventricular ejection fraction, and increased ventricular septum were independent risk factors for preoperative myocardial injury in patients with gastrointestinal tumors. The proportion of clinical symptoms in gastrointestinal tumor patients with myocardial injury was low, indicating the necessity to closely monitor the cardiac status of individuals with gastrointestinal tumors.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-023-03086-1.
Keywords: Gastrointestinal tumor, Myocardial injury, High-sensitive cardiac troponin I, Risk factor
Introduction
Gastrointestinal tumor has become one of the most prevalent tumors around the world, affecting millions of people worldwide [1, 2]. Early diagnosis and development in tumor treatment have greatly improved gastrointestinal tumor patients’ overall survival rate. However, these patients often die of cardiovascular disease rather than recurrence of their tumor [3].
Hs-cTnI (high-sensitive cardiac troponin I) is a index with high specificity and sensitivity for the detection of myocardial injury, which can reflect the myocardial injury of non-cardiogenic diseases. Elevated levels of hs-cTnI is associated with higher mortality in non-cardiac hospitalized patients [4–6], including patients with gastrointestinal tumors [7]. Taken together, myocardial injury should be given high priority [8]. Considering the shared cardiovascular risks between tumors and cardiovascular disease [9], inflammatory states associated with malignancies, and cardiotoxic effects of cancer therapy, gastrointestinal tumors complicated with myocardial injury may be common. Most studies focus on the monitoring of myocardial injury during the treatment of gastrointestinal tumors, ignoring the possible myocardial injury caused by the disease itself. It may be of great significance to clarify risk factors of myocardial injury before surgery for the necessity of taking preventive intervention. Furthermore, preoperative intervention of myocardial injury may bring a better prognosis to patients.
However, to the best of our knowledge, no studies have reported the incidence and risk factors of myocardial injury in patients with gastrointestinal tumors. This is a retrospective study to investigate the risk factors leading to myocardial injury in patients and thus screening out patients with high-risk gastrointestinal tumors complicated with myocardial injury.
Methods
Study design and participants
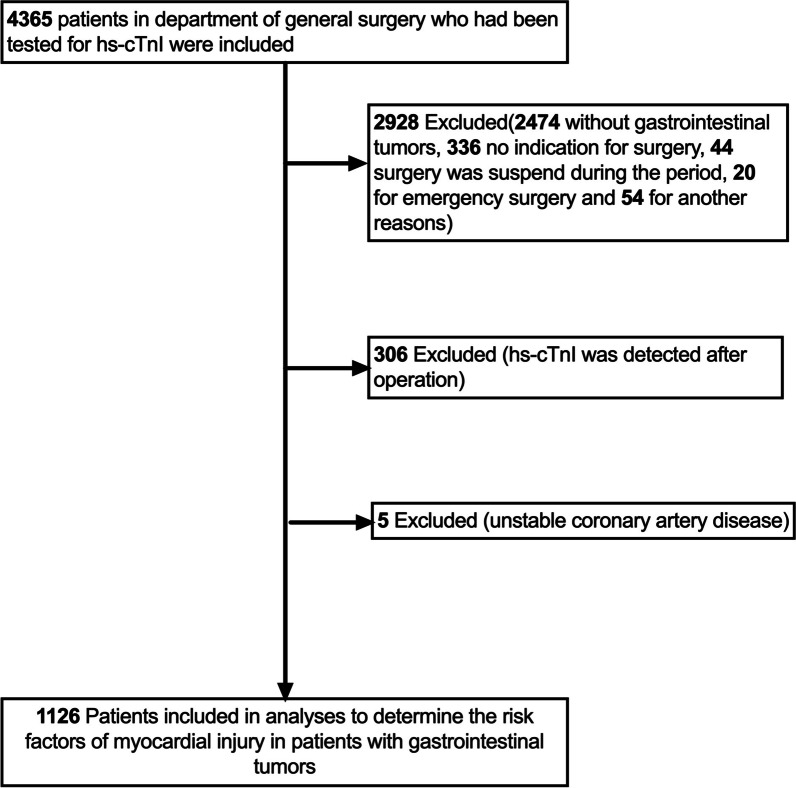
This was a single-center retrospective study. Patients who admitted to the Sixth Affiliated Hospital of Sun Yat-sen University from May 2018 to June 2020 and had been tested for hs-cTnI were screened. 1126 consecutive patients who compliance with inclusion criteria and were finally enrolled (Fig. 1).
Fig. 1.
Flow diagram that shows the process of enrollment and exclusion. Hs-cTnI, high sensitive-cardiac troponin I
Patients with gastrointestinal tumors (age ≥ 18 years old) who underwent tumor related surgery under general anesthesia and completed hs-cTnI testing at admission within 7 days before surgery were consecutively enrolled. Exclusion criteria were emergency surgery, failure to received tumor related surgery, and clinical evidence of acute coronary syndrome (typical angina pectoris with or without ST-segment elevation electrocardiograph alteration) in the medical history of preoperative assessment. The database of this study comes from the study published in EClinicalMedicine in August 2021 [7].
This study has been approved by the Medical Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (2021ZSLYEC-296).
Study definitions
History of coronary heart disease, defined as undergoing previous bypass surgery, coronary intervention, myocardial infarction, or meeting the guidelines definition [10, 11]. Myocardial infarction was diagnosed using the universal definition of myocardial infarction [12].
Lee index (revised cardiac index) was calculated as follows: high-risk surgical type, history of ischemic heart disease, history of congestive heart failure, history of cerebrovascular disease, history of insulin therapy for diabetes mellitus, and preoperative serum creatinine > 2.0 mg/dL (176.8 μmol/L) accounting for 1 point each [13].
Data collection
We analyzed the data of the most recent examination before surgery during hospitalization. Data including patients’ characteristics, laboratory index and echocardiographic parameters at baseline, comorbidities, premedication and outcome (the ICU detention time) were all collected from medical records. Patients were divided into the myocardial injury group and the non-myocardial injury group according to the hs-cTnI elevation (≥ 0.028 ng/ml) at admission.
Blood samples were collected within 7 days before surgery. The samples were collected from the antecubital vein, stored in additive-free test tubes, and processed immediately. Using centrifugation to separate serum and stored it at -70℃ until analysis. On an analyzer, hs-cTnI was quantified using a high-sensitivity electrochemiluminescence-immunoassay (ABBOTT, Architect i1000SR). The 99th percentile reference value of hs-cTnI was 0·028 ng/ml. All of the tests were carried out at Sun Yat-sen University’s Sixth Affiliated Hospital's clinical laboratory.
All echocardiographic examinations were conducted during hospitalization prior to surgery. The images were recorded using a digital ultrasound device and archived according to the requirements of the American Society of Echocardiography (GE vivid E9).
Statistical analysis
We used SPSS22.0 statistical software to process all analyses. Continuous variables are expressed as means ± standard deviation or median [25%, 75%], and classification variables are expressed as frequency (percentage). Continuous variables were compared using the independent T-test or paired sample T test or Mann–Whitney U test, and categorical variables were compared using the Chi-square test or Fisher's exact test where appropriate. Odds ratios for candidate variables were calculated by univariate logistic regression analyses. The indexes with significant differences were analyzed by multivariate logistic regression, and a P-value < 0.05 was considered statistically significant. Missing values were excluded from the relevant analysis.
Results
Baseline characteristics in the myocardial injury group and non-myocardial injury group
The final sample size consisted of 1126 patients (Fig. 1). Of the 1126 patients (35.8% females; mean age 62.6 years) enrolled in this study, 78 (6.93%) patients had elevated hs-cTnI (Hs-cTnI ≥ 0.028), which is defined as myocardial injury group. The main surgical sites included: gastric (109, 9.7%), colorectum (994, 88.3%), intestinal (20, 1.8%), esophageal (1, 0.1%) and anal tube (2, 0.2%). In this study, there were 7 patients presented with clinical symptoms in the myocardial injury group (chest discomfort in 4 cases, non-compressive precordial chest pain in 1 case, dyspnea in 2 cases).
As shown in Table 1 and 2, the mean age of the 78 gastrointestinal tumor patients with myocardial injury was 71.0 ± 11.1 years, which is higher than that in non-myocardial injury group (P < 0.001). Compared with patients in the non-myocardial injury group, patients in the myocardial injury group have a higher prevalence of cardiovascular risk factors (including senior age and higher proportion of smoking), a higher prevalence of comorbidities (such as previous coronary artery disease, hypertension, atrium fibrillation and diabetes, and a higher rate of premedication (such as anticoagulation, β-blocker, Angiotensin-converting enzyme inhibitor/Angiotens in II receptor blocker, and diuretic) (all with P-value < 0.05). As compared with non-myocardial injury group, patients in the myocardial injury group also presented with a higher revised cardiac risk index (Lee index), higher neutrophil granulocyte ratio, lower hemoglobin, and higher likelihood of impaired cardiac structure and function (all with P-value < 0.05). In addition, 6.4% of patients from the myocardial injury group take statins, which is relatively higher than that in non-myocardial injury group. There was no statistical difference in the proportion of receiving preoperative chemotherapy between the two groups. (The chemotherapy regimen was provided in Additional file 1).
Table 1.
Baseline characteristics
| All | All patients | n1 | Non-myocardial injury group (n = 1048) | n2 | myocardial injury group (n = 78) | P* | |
|---|---|---|---|---|---|---|---|
| Gender (%) | 723(64.2%) | 667(63.6%) | 56(71.8%) | 0.147 | |||
| Age (years) | 1126 | 62.6 ± 11.9 | 1048 | 62.0 ± 11.7 | 78 | 71.0 ± 11.1 | < 0.001 |
| BMI (kg/m2) | 1109 | 23.4 ± 3.4 | 1034 | 22.4 ± 3.3 | 75 | 22.3 ± 3.8 | 0.748 |
| History | |||||||
| Smoking | 1126 | 0.003 | |||||
| Never smoking | 996(88.5%) | 928(88.5%) | 68(87.2%) | ||||
| Used to smoke | 115(10.2%) | 110(10.5%) | 5(6.4%) | ||||
| Current smoking | 15(1.3%) | 10(1.0%) | 5(6.4%) | ||||
| CAD | 85(7.5%) | 65(6.2%) | 20(25%) | < 0.001 | |||
| PCI history | 33(2.9%) | 27(2.6%) | 6(7.7%) | 0.025 | |||
| CABG history | 1(0.1%) | 0 | 1(1.3%) | 0.069 | |||
| Hypertension | 248(22.0%) | 215(20.5%) | 33(42.3%) | < 0.001 | |||
| Atrial fibrillation | 11(0.9%) | 7(0.7%) | 4(5.1%) | 0.005 | |||
| Diabetes mellitus | 113(10.0%) | 98(9.4%) | 15(19.2%) | 0.005 | |||
| Insulin dependent | 17(1.5%) | 11(1.0%) | 6(7.7%) | < 0.001 | |||
| Chronic renal failure | 6(0.5%) | 5(0.5%) | 1(0.3%) | 0.351 | |||
| Dialysis | 1(0.1%) | 0 | 1(1.3%) | 0.069 | |||
| Medical treatment | 1126 | ||||||
| Aspirin | 24(2.1%) | 21(2.0%) | 3(3.8%) | 0.496 | |||
| Clopidogrel | 29(2.6%) | 25(2.4%) | 4(5.1%) | 0.269 | |||
| Anticoagulation | 22(2.0%) | 15(1.4%) | 7(9.0%) | < 0.001 | |||
| β-blocker | 34(3.0%) | 27(2.6%) | 7(9.0%) | 0.004 | |||
| ACE inhibitor/ARB | 75(6.7%) | 64(6.1%) | 11(14.1%) | 0.006 | |||
| ARNI | 2(0.2%) | 2(0.2%) | 0 | 1 | |||
| CCB | 110(9.8%) | 98(9.4%) | 12(15.4%) | 0.083 | |||
| Statins | 40(3.6%) | 35(3.3%) | 5(6.4%) | 0.273 | |||
| Diuretic | 20(1.8%) | 15(1.4%) | 5(6.4%) | 0.006 | |||
| Metformin | 41(3.6%) | 35(3.3%) | 6(7.7%) | 0.096 | |||
| Chemotherapy | 148(13.1%) | 136(13.0%) | 12(15.4%) | 0.544 | |||
| Radiation therapy | 21(1.9%) | 21(2.0%) | 0 | 0.408 | |||
| LEE Index | 1126 | < 0.001 | |||||
| 0 | 993(88.2%) | 943(90.0%) | 50(64.1%) | ||||
| 1 | 112(9.9%) | 90(8.6%) | 22(28.2%) | ||||
| ≥ 2 | 21(1.9%) | 15(1.4%) | 6(7.7%) | ||||
| NYHA class II-IV | 14(1.2%) | 10(1.0%) | 4(5.1%) | 0.007 | |||
| Radical operation | 1006(89.3%) | 951(90.7%) | 55(71.4%) | < 0.001 | |||
| HR (beats per min) | 1125 | 80.4 ± 12.9 | 1047 | 80.4 ± 13.0 | 78 | 80.1 ± 11.9 | 0.868 |
| SBP (mmHg) | 1124 | 126.1 ± 18.3 | 1046 | 125.8 ± 18.1 | 78 | 130.7 ± 20.0 | 0.021 |
| DBP (mmHg) | 1124 | 76.9 ± 10.7 | 1046 | 77.0 ± 10.7 | 78 | 75.4 ± 10.4 | 0.184 |
Continuous variables are expressed as means ± standard deviation. Categorical variables are expressed as frequencies (percentage)
BMI, body mass index; CAD, coronary artery disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; ANRI, angiotensin receptor neurolysin inhibitors; NYHA, New York Heart Association; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure
*Significance of the differences between the patients with and without myocardial injury
Table 2.
Laboratory index, echocardiographic parameters on admission
| All | All patients | n1 | Non-myocardial injury group (n = 1048) | n2 | myocardial injury group (n = 78) | P | |
|---|---|---|---|---|---|---|---|
| HB (g/L) | 1124 | 119.2 ± 24.8 | 1046 | 119.7 ± 24.9 | 78 | 111.9 ± 22.7 | 0.007 |
| RDW (%) | 1121 | 15.3 ± 5.7 | 1043 | 15.3 ± 5.8 | 78 | 15.8 ± 3.9 | 0.44 |
| PDW (%) | 1105 | 11.7 ± 2.5 | 1027 | 11.7 ± 2.5 | 78 | 12.1 ± 2.9 | 0.157 |
| WBC(× 10^9/L) | 1124 | 6.6 ± 3.2 | 1046 | 6.5 ± 3.2 | 78 | 6.9 ± 3.0 | 0.318 |
| NEUR (%) | 1124 | 29.9 ± 30.8 | 1046 | 28.3 ± 30.5 | 78 | 50.7 ± 27.1 | < 0.001 |
| PCT (ng/ml) | 138 | 0.05 ± 0.62 | 136 | 0.05 ± 0.6 | 2 | 0.06 ± 0.1 | 0.989 |
| CRP (mg/L) | 105 | 5.5 ± 18.2 | 1001 | 5.6 ± 18.0 | 49 | 3.6 ± 21.8 | 0.453 |
| Cr (μmol/L) | 1115 | 78.2 ± 41.7 | 1038 | 76.8 ± 24.2 | 77 | 97.9 ± 130.6 | 0.161 |
| LDL-C (mmol/L) | 1078 | 3.2 ± 0.9 | 1006 | 3.2 ± 0.9 | 72 | 2.8 ± 0.7 | < 0.001 |
| AST (U/L) | 1119 | 22.1 ± 13.5 | 1042 | 21.8 ± 13.4 | 77 | 25.4 ± 14.5 | 0.041 |
| ALT (U/L) | 1119 | 18.9 ± 17.8 | 1042 | 18.9 ± 18.1 | 77 | 18.3 ± 13.4 | 0.779 |
| TBIL (g/L) | 1101 | 12.4 ± 8.1 | 1025 | 12.4 ± 8.2 | 76 | 12.1 ± 5.6 | 0.736 |
| DBIL (g/L) | 1101 | 2.7 ± 4.2 | 1025 | 2.7 ± 4.3 | 76 | 3.2 ± 2.9 | 0.336 |
| CKMB (U/L) | 1034 | 16.5 ± 23.4 | 961 | 16.8 ± 24.2 | 73 | 13.3 ± 6.6 | 0.002 |
| BNP (pg/ml) | 86 | 204.7 ± 474.7 | 63 | 157.5 ± 434.0 | 23 | 334.1 ± 562.2 | 0.127 |
| MYO (ng/ml) | 1126 | 44.5 ± 75.9 | 1048 | 40.8 ± 40.2 | 78 | 94.2 ± 244.2 | 0.058 |
| D-dimer (mg/L) | 630 | 0.9 ± 1.5 | 581 | 0.9 ± 1.3 | 49 | 1.7 ± 2.9 | 0.066 |
| CEA (ng/ml) | 1107 | 31.8 ± 302.9 | 1032 | 31.3 ± 310.6 | 75 | 38.2 ± 164.0 | 0.848 |
| CA125 (U/L) | 1111 | 23.6 ± 70.0 | 1036 | 19.6 ± 34.3 | 75 | 79.2 ± 231.7 | 0.029 |
| CA199 (U/L) | 1101 | 243.6 ± 3814.0 | 1026 | 141.7 ± 1266.9 | 75 | 1637.7 ± 13,852.4 | 0.353 |
| LVEF (%) | 1015 | 66.6 ± 6.2 | 942 | 68.8 ± 5.9 | 73 | 63.4 ± 8.1 | < 0.001 |
| LVDd (mm) | 1015 | 44.2 ± 5.5 | 942 | 44.1 ± 5.4 | 73 | 45.9 ± 6.0 | 0.006 |
| LA (mm) | 1015 | 30.5 ± 4.7 | 942 | 30.3 ± 4.5 | 73 | 33.1 ± 5.7 | < 0.001 |
| IVS (mm) | 1015 | 9.5 ± 1.6 | 942 | 9.4 ± 1.5 | 73 | 10.4 ± 1.9 | < 0.001 |
| LVPW (mm) | 1015 | 9.2 ± 1.4 | 942 | 9.1 ± 1.3 | 73 | 9.9 ± 1.7 | < 0.001 |
Data are expressed as means ± standard deviation
HB, hemoglobin; WBC, white blood cell; NEUR, neutrophil granulocyte ratio; PCT, procalcitonin; CRP, C-reactive protein; LDL, low-density lipoprotein; ALT, alanine aminotransferase; AST, aspartate transaminase; TBIL, total bilirubin; DBIL, direct bilirubin; CKMB, creatine phosphokinase-MB; BNP, brain natriuretic peptide; MYO, myoglobin; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA199, carbohydrate antigen 199; LVEF, left ventricular ejection fraction; LVDd, left ventricular end-diastolic diameter; LA, left atrium; IVS, interventricular septum; LVPW, left ventricular posterior wall. P < 0.001
The influence of myocardial injury on the outcome of patients with gastrointestinal tumor
Moreover, there was a statistical significance in ICU admission between the myocardial injury group and the non-myocardial injury group (22.1% vs. 4.2%, P < 0.001), and there was a trend of statistical significance in the ICU detention time (1[1, 3] vs. 2[1, 10], P = 0.064) between the two groups.
Risk factors for myocardial injury in patients with gastrointestinal tumor
As shown in Table 3, univariate analysis revealed that advanced age, higher Lee index, increased systolic blood pressure, increased neutrophil granulocyte ratio, decreased hemoglobin, increased aspartate transaminase, higher CA125, decreased LVEF, increased left ventricular end-diastolic diameter, increased left atrium diameter, as well as increased interventricular septum thickness and left ventricular posterior wall thickness were risk factors for myocardial injury in patients with gastrointestinal tumor. Preoperative chemotherapy is not a significant risk factor in preoperative myocardial injury in patients with gastrointestinal tumors (OR = 1.219 [0.642–2.314], P = 0.544).
Table 3.
Univariate logistic regression analysis of risk factors for myocardial injury
| OR | 95%CI | P | |
|---|---|---|---|
| Age (years) | 1.079 | 1.054–1.105 | < 0.001 |
| LEE Index | 3.384 | 2.303–4.973 | < 0.001 |
| SBP (mmHg) | 1.014 | 1.002–1.027 | 0.021 |
| HB (g/L) | 0.988 | 0.979–0.997 | 0.008 |
| NEUR (%) | 1.025 | 1.017–1.034 | < 0.001 |
| AST (U/L) | 1.012 | 1.000–1.024 | 0.043 |
| CA-125 | 1.008 | 1.004–1.012 | < 0.001 |
| LVEF (%) | 0.927 | 0.897–0.959 | < 0.001 |
| LVDd (mm) | 1.065 | 1.019–1.114 | 0.006 |
| LA (mm) | 1.123 | 1.072–1.177 | < 0.001 |
| IVS (mm) | 1.415 | 1.232–1.625 | < 0.001 |
| LVPW (mm) | 1.524 | 1.293–1.797 | < 0.001 |
As shown in Table 4, advanced age, increased Lee index score, increased neutrophil granulocyte ratio, decreased LVEF and increased interventricular septum were still independent risk factors for myocardial injury in multivariate logistic regression analysis. As compared with patients with Lee index = 0, OR value (95% CI) in patients with Lee index = 1 and 2 were 2.559 (1.335–4.908, P = 0.005) and 2.378 (0.686–8.238, P = 0.172), respectively.
Table 4.
Multivariate logistic regression analysis of risk factors for myocardial injury
| OR | 95%CI | P | |
|---|---|---|---|
| Age (years) | 1.065 | 1.036–1.096 | < 0.001 |
| NEUR (%) | 1.025 | 1.015–1.035 | < 0.001 |
| LVEF (%) | 0.936 | 0.901–0.972 | < 0.001 |
| IVS (mm) | 1.401 | 1.194–1.644 | < 0.001 |
| LEE Index | 0.012 | ||
| LEE1vs0 | 2.559 | 1.335–4.908 | 0.005 |
Discussion
We found in the present study 78 cases (6.93%) of 1126 patients with gastrointestinal tumors were complicated with myocardial injury. Advanced age, increased Lee index score, elevated neutrophil granulocyte ratio, decreased LVEF, and increased ventricular septum thickening were identified as independent risk factors for gastrointestinal tumor complicated with myocardial injury.
Previous studies reported myocardial injury occurs frequently (42/586, 7.17%) in patients undergoing colorectal tumor surgery in an enhanced recovery after surgery protocol [6]. We reported incidence rate of gastrointestinal tumor combined with myocardial injury before surgery, which is 6.93%. The interpretation of the results needs to be cautious since only the patients who received surgery were selected. High-risk patients may have been excluded. This may underestimate the incidence of myocardial injury in patients with gastrointestinal tumors. Further prospective multicenter studies are needed to confirm this speculation.
Our study indicates that advanced age is a risk factor for preoperative myocardial injury in patients with gastrointestinal tumors. This is different from previous reports which showed age is not a risk factor for upper gastrointestinal bleeding with simultaneous myocardial injury [14]. However, there was a study shown that elderly people are more likely to suffer from hypertension, diabetes, peripheral vascular disease, chronic heart failure, and renal failure [15]. Our study is consistent with a previous study which demonstrated advanced age is a major risk factor for cardiovascular disease [16]. The mechanisms probably as follows: firstly, aging reduced mitochondrial content and progressively slower stress response to ischemia, contributing to myocardial injury; secondly, autophagy may decrease in aging myocardium as a cellular protective cycling mechanism, leading to cardiac dysfunction and myocardial injury [17, 18]. Lee index was widely applied to identify patients at higher risk for perioperative complications or myocardial injury in patients undergoing non-cardiac surgery [13, 19]. Our study demonstrated that Lee index is an independent risk factor for preoperative myocardial injury in patients with gastrointestinal tumors. Our study seems to consistent with a previous study which reported that upper gastrointestinal bleeding patients with more than three cardiac risk factors comprised a high-risk group for simultaneously developing myocardial injury [14]. Tumor patients complicated with diabetes are in chronic hyperglycemic states, which can simultaneously cause microangiopathy in the cardiovascular system, leading to punctate necrosis of the myocardium. Elevated hs-cTnI in patients with chronic kidney disease may be associated with its reduced excretion rate, which causing myocardial damage due to toxin accumulation in the body. This mechanism may explain the relationship between Lee index and myocardial injury.
Inflammation is recognized as a prominent feature of tumor progression [20]. Studies have shown that about 20–40% of gastrointestinal cancer patients have systemic inflammation before surgery, which is one of the indicators of poor prognosis before surgery [21]. Systemic inflammation may promote the progression of myocardial injury during the perioperative period [22]. Our findings indicate that the neutrophil granulocyte ratio is an independent risk factor for preoperative myocardial injury in patients with gastrointestinal tumors. Feldstein et al. suggested that inflammation may be the mechanism for myocardial injury [23], which is consistent with our findings.
We found that LVEF reduction and ventricular septum thickening were independent risk factors for gastrointestinal tumors complicated with myocardial injury. Previous studies have shown that hs-cTnI concentration is related to left ventricular diastolic dysfunction as suggested by echocardiography [24]. Therefore, echocardiography indicators such as LVEF and ventricular septal thickness may reflect the severity of myocardial injury, which is in line with our research results.
We found that there was a trend of statistical significance in the ICU detention time between the myocardial injury group and the non-myocardial injury group, which is consistent with the results of Tota-Maharaj et al.’ s study [25]. The possible reasons are as follows: (1) The status of tumor patients complicated with myocardial injury was more complex, requiring additional cardiac examination, monitoring and intensive care.
There are several limitations in this study. Firstly, this study is a retrospective single-center study, and the inclusion of gastrointestinal tumor patients undergoing surgical treatment does not represent the incidence of myocardial injury in all patients with gastrointestinal tumors. Prospective multicenter research is warranted in the future. However, our research suggests that gastrointestinal tumor is sometimes complicated with myocardial injury, which cannot be ignored in clinical practice. Secondly, there was no statistical significance between Lee index = 2 and Lee index = 0, which may be due to the small sample size of gastrointestinal tumor patients with Lee index ≥ 2. Thirdly, in order to better assess the cardiac status of gastrointestinal tumor patients and take necessary intervention before surgery, our present study focuses on the risk factors for preoperative myocardial injury. Therefore, risk factors for postoperative myocardial injury were not investigated in the present study.
Conclusions
In conclusion, advanced age, increased Lee index, increased neutrophil granulocyte ratio, decreased left ventricular ejection fraction, and enlarged ventricular septum were independent risk factors for preoperative myocardial injury in patients with gastrointestinal tumors. The proportion of clinical symptoms in gastrointestinal tumor patients with myocardial injury was low, indicating the necessity to closely monitor the cardiac status of individuals with gastrointestinal tumors in preoperative period.
Supplementary Information
Additional file 1. Chemotherapy information that shows the chemotherapy regimen and proportion of patients in each group.
Acknowledgements
None.
Author contributions
WZ, YZ contributed to the conception and design of this manuscript. JS, SY, HP, SH acquired data. JX, ZC, LZ performed statistical analyses and interpreted the data, SY, SC drafted the manuscript, WZ and JW modified the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Fundamental Research Funds for the Central Universities (19ykpy10) and The National Natural Science Foundation of China (Grant No. 81400301).
Availability of data and materials
The data underlying the results presented in this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The study followed the principles of the Declaration of Helsinki and was approved by the Medical Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (2021ZSLYEC-296). All participants in the study provided Informed consent after being fully informed of the purpose of the research.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shuqi Yu and Shiyao Cheng have contributed equally to this work
Contributor Information
Yitao Zhang, Email: zhangyt73@mail.sysu.edu.cn.
Weijie Zeng, Email: zengweijie@mail.sysu.edu.cn.
References
- 1.Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021;14(10):101174. doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Giza DE, Iliescu G, Hassan S, et al. Cancer as a risk factor for cardiovascular disease. Curr Oncol Rep. 2017;19(6):39. doi: 10.1007/s11912-017-0601-x. [DOI] [PubMed] [Google Scholar]
- 4.Kelley WE, Januzzi JL, Christenson RH. Increases of cardiac troponin in conditions other than acute coronary syndrome and heart failure. Clin Chem. 2009;55(12):2098–2112. doi: 10.1373/clinchem.2009.130799. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed AN, Blonde K, Hackam D, et al. Prognostic significance of elevated troponin in non-cardiac hospitalized patients: a systematic review and meta-analysis. Ann Med. 2014;46(8):653–663. doi: 10.3109/07853890.2014.959558. [DOI] [PubMed] [Google Scholar]
- 6.Zahid JA, Orhan A, Ekeloef S, et al. Myocardial injury after colorectal cancer surgery and postoperative 90-day mortality and morbidity: a retrospective cohort study. Dis Colon Rectum. 2021;64(12):1531–1541. doi: 10.1097/DCR.0000000000002061. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Xue J, Zhou L, et al. The predictive value of high-sensitive troponin I for perioperative risk in patients undergoing gastrointestinal tumor surgery. EClinicalMedicine. 2021;40:101128. doi: 10.1016/j.eclinm.2021.101128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang S, Peng W, Yang N, et al. Myocardial injury in elderly patients after abdominal surgery. Aging Clin Exp Res. 2018;30(10):1217–1223. doi: 10.1007/s40520-018-0908-y. [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Cheng Y, Luo D, et al. Association between cardiovascular risk factors and colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. EClinicalMedicine. 2021;34:100794. doi: 10.1016/j.eclinm.2021.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European society of cardiology. Eur Heart J. 2013;34(38):2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 11.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 13.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi: 10.1161/01.CIR.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 14.Wu IC, Yu FJ, Chou JJ, et al. Predictive risk factors for upper gastrointestinal bleeding with simultaneous myocardial injury. Kaohsiung J Med Sci. 2007;23(1):8–16. doi: 10.1016/S1607-551X(09)70368-7. [DOI] [PubMed] [Google Scholar]
- 15.Plante GE. Impact of aging on the body's vascular system. Metabolism. 2003;52(10 Suppl 2):31–35. doi: 10.1016/S0026-0495(03)00299-3. [DOI] [PubMed] [Google Scholar]
- 16.Hamczyk MR, del Campo L, Andrés V. Aging in the cardiovascular system: lessons from Hutchinson-Gilford progeria syndrome. Annu Rev Physiol. 2018;80:27–48. doi: 10.1146/annurev-physiol-021317-121454. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Ren J. Aging as a risk factor for cardiac surgery: Blunted ischemic-reperfusion stress response? J Card Surg. 2021;36(10):3641–3642. doi: 10.1111/jocs.15806. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira AN, Yanagawa B, Quan A, et al. Human cardiac ischemia-reperfusion injury: blunted stress response with age. J Card Surg. 2021;36(10):3643–3651. doi: 10.1111/jocs.15807. [DOI] [PubMed] [Google Scholar]
- 19.Roshanov PS, Sessler DI, Chow CK, et al. Predicting myocardial injury and other cardiac complications after elective noncardiac surgery with the revised cardiac risk index: the VISION study. Can J Cardiol. 2021;37(8):1215–1224. doi: 10.1016/j.cjca.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Tuomisto AE, Mäkinen MJ, Väyrynen JP. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroenterol. 2019;25(31):4383–4404. doi: 10.3748/wjg.v25.i31.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ackland GL, Abbott TEF, Cain D, et al. Preoperative systemic inflammation and perioperative myocardial injury: prospective observational multicentre cohort study of patients undergoing non-cardiac surgery. Br J Anaesth. 2019;122(2):180–187. doi: 10.1016/j.bja.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. 2020;383(4):334–46. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landesberg G, Jaffe AS, Gilon D, et al. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014;42(4):790–800. doi: 10.1097/CCM.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 25.Tota-Maharaj R, Perera B, Murray J, et al. Impact of troponin testing in noncardiac admissions. Crit Pathw Cardiol. 2014;13(4):147–151. doi: 10.1097/HPC.0000000000000027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Chemotherapy information that shows the chemotherapy regimen and proportion of patients in each group.
Data Availability Statement
The data underlying the results presented in this study are available from the corresponding author upon reasonable request.