Abstract
Colorectal cancer mortality rate and highly altered proteins from the Wnt/β-catenin pathway increase the scientific community’s interest in finding alternatives for prevention and treatment. This study aims to determine the biological effect of chlorogenic acid (CGA) on two colorectal cancer cell lines, HT-29 and SW480, and its interactions with β-catenin and LRP6 to elucidate a possible modulatory mechanism on the Wnt/β-catenin pathway. These effects were determined by propidium iodide and DiOC6 for mitochondrial membrane permeability, MitoTracker Red for mitochondrial ROS production, DNA content for cell distribution on cell cycle phases, and molecular docking for protein–ligand interactions and binding affinity. Here, it was found that CGA at 2000 µM significantly affects cell viability and causes DNA fragmentation in SW480 cells rather than in HT-29 cells, but in both cell lines, it induces ROS production. Additionally, CGA has similar affinity and interactions for LRP6 as niclosamide but has a higher affinity for both β-catenin sites than C2 and iCRT14. These results suggest a possible modulatory role of CGA over the Wnt/β-catenin pathway in colorectal cancer.
Keywords: chlorogenic acid, colorectal cancer, apoptosis, proliferation, β-catenin, LRP6
1. Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related death worldwide and the fourth with more incidence [1]. Like other types of cancer, the primary treatment for CRC is surgery; nevertheless, most patients are asymptomatic until the tumor progresses and must be treated with a combination of chemotherapy, radiotherapy, and biotherapy [2]. Despite advances in prevention, screening, and chemotherapy, the 5-year survival rate in CRC is about 11% of all cancer [3]. For this reason, developing new treatment regimens for managing these patients is mandatory [4].
Chlorogenic acid (CGA) is a polyphenol widely distributed in nature. It is an ester of caffeic acid with quinic acid, whose antiproliferative effect in CRC has been demonstrated [5]. Previous studies using in vitro colon cancer models have shown that CGA decreases viability and modulates the proliferative and migratory capabilities of Caco-2 [6], HT-29, SW480, and SW620 [7,8] cells at EC50 758 uM and IC50 8114 μg/mL, 686.6 μg/mL, and 828.6 μg/mL, respectively. Likewise, it has been shown that CGA diminishes the viability of cancer cells derived from liver [9,10,11], breast [12,13], lung [14,15], blood [16,17], bone [18], and kidney [19] tissues, suggesting the potential of CGA for the modulation of biological mechanisms involved in cancer cell survival.
Wnt/β-catenin is a highly conservative signaling pathway that plays a crucial role in embryonic development and regulates the self-renewal and homeostasis of various adult tissues [2]. More than 94% of CRC cases have at least an altered protein from the Wnt/β-catenin signaling pathway by a genetic and epigenetic mechanism [20]. However, early APC mutations are acquired in over 80% of colon cancer patients, leading to the cytosolic accumulation of β-catenin, an intracellular signal transducer in transcriptional regulation that, in combination with TCF/Lef1, promotes proliferation and inhibits apoptosis [21]. Moreover, recently, we reported the modulatory effect of CGA treatment ong SW480 and HT-29 cell, by decreasing the transcriptional activation of the Wnt pathway at similar proportion as the selective pathway inhibitor iCRT14 [8].
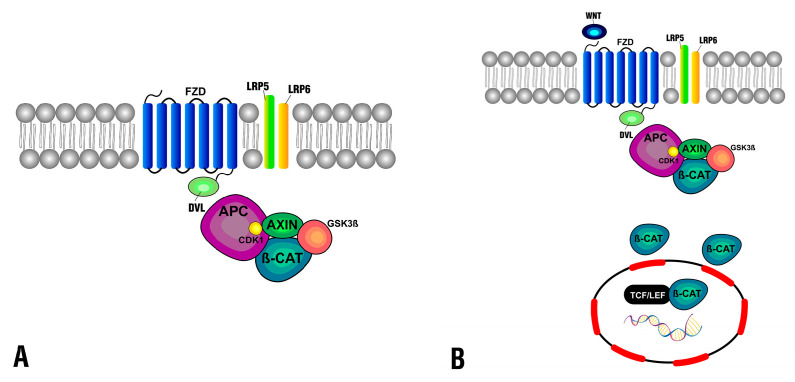
In normal physiological conditions, β-catenin degrades in the cytoplasm by the destruction complex consisting of axis inhibition protein (AXIN), adenomatosis polyposis coli (APC), casein kinase 1 (CDK1), and glycogen synthase kinase 3β (GSK3β) (Figure 1A). As a consequence of Wnt ligands binding to the Frizzled protein receptor at the membrane and its coreceptor LRP5/6, the destruction complex function becomes displaced, which leads to the accumulation of β-catenin and its translocation to the nucleus (Figure 1B), consequently triggering the CRC [22].
Figure 1.
Canonical Wnt/β-catenin signaling pathway. (A) Regulated state when the destruction complex controls β-catenin levels at the cytoplasm. (B) Dysregulated state where the presence of Wnt ligands and the interaction with membrane receptors displace the destruction complex and β-catenin accumulates at the cytoplasm, and then translocated to the nucleus.
LRP6 possesses extracellular and intracellular domains. The extracellular domain is necessary for the interaction with Wnt ligands and also with Dickkopf-related protein 1 (DKK1) [23], a protein that antagonizes the P3E3P4E4 domain by competitive binding, disrupting the initiatory complex Frizzled–LRP6 [24]. For this reason, inhibiting LRP6 can be a potential solution for diseases such as osteoporosis, Alzheimer, cancer, and neurodegeneration [23]. It has been found that niclosamide is a potent Wnt/β-catenin inhibitor by inducing LRP6 degradation in HEK293 cells [25]. Furthermore, it has been observed that niclosamide suppresses the growth of HCT116, LoVo, SW620, and HT-29 cell lines [26], and additionally induces cell apoptosis [27].
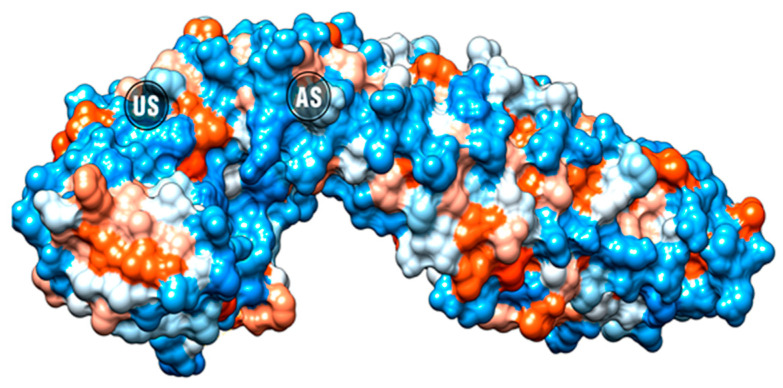
The WNT pathway inhibition could also be assessed by blocking the most downstream component, the interaction between β-catenin and T-cell factor 4 (TCF4) at the nucleus. This inhibition can be effectively achieved regardless of the mutations in the upstream components [28]. iCRT3, iCRT5, and iCRT14 effectively inhibit β-catenin–TCF4 interaction while allowing β-catenin binding with E-cadherin at the cell–cell adherens junctions, which is essential for the prevention of metastasis [29]. β-catenin has two binding sites (Figure 2), the well-known union site (US) between β-catenin and TCF4 [30] and the recently reported allosteric site (AS) [31].
Figure 2.
β-catenin surface with the position of the allosteric site (AS) and the TCF4 binding site (US) highlighted.
The CRC model selected for this research consists of two cell lines with different truncating mutations in the APC gene. In SW480, the mutation at the 1338 residue changes the APC domain that interacts with β-catenin for ubiquitination and degradation, while in HT-29, the truncating mutation in the 1555 residue maintains the regulatory effect of the APC protein over β-catenin [32,33,34].
Proliferation and apoptosis are processes highly involved in cancer development and important to cancer therapy [35,36]. Despite the multiple studies evaluating the proliferation and apoptosis in colorectal cell lines, to the best of our knowledge, there are no available reports comparing the effects of CGA on SW480 and HT-29 cell lines, in which differences in the Wnt/β-catenin pathway have been reported. Although there are studies describing that CGA regulates Wnt/β-catenin signaling [37,38], none of them discriminate which targets are involved in the mechanism.
This study aims to determine the effect of CGA in proliferation and apoptosis on two colorectal cancer cell lines and its interactions with β-catenin and LRP6 to elucidate a possible modulatory mechanism of CGA on CRC in the context of Wnt/β-catenin pathway.
2. Results
2.1. CGA Treatment Affects Cell Viability
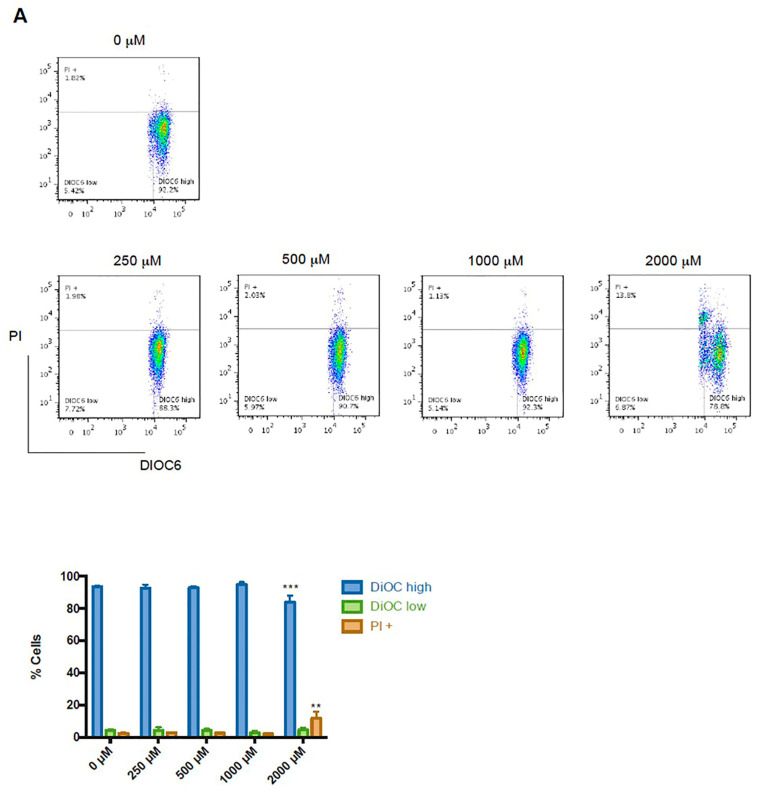
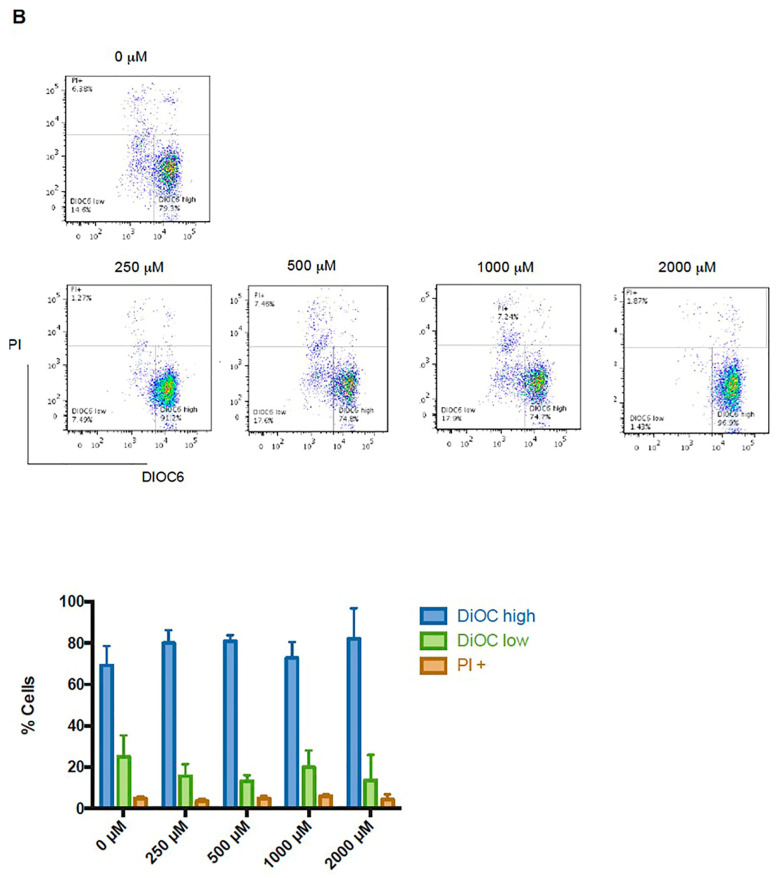
Mitochondrial polarization changes and loss of cell membrane integrity are common indicators of cell death. SW480 and HT-29 colon cancer cells were treated with different concentrations of CGA to determine the cytotoxic effect. Flow cytometry was used to quantify the DiOC6 retention and PI uptake. In SW480 cells, 2000 µM of CGA induced a significant decrease in DiOC6 high population with a related increase in PI uptake (Figure 3A). These results indicate a reduction in cell viability because mitochondrial dysfunction is directly involved in the intrinsic apoptotic pathway. Under the same conditions, HT-29 cells do not exhibit a significant difference (Figure 3B).
Figure 3.
Cytotoxic effect of CGA in colon cancer cells. (A) SW480 and (B) HT-29 cells were treated with CGA at indicated concentrations. Mitochondrial membrane potential was measured with DiOC6 and cell membrane by PI intake and analyzed using flow cytometry. DiOC6 high: live cells with high membrane polarization; DiOC6 low and PI negative: cells with membrane depolarization; PI+: dead cells. The figure shows a representative histogram of flow cytometry analysis and bar graphs for quantification for each cell line. Two-way ANOVA for DiOC high, DiOC low, and PI+ populations shows the difference concerning untreated cells, where ** p ≤ 0.01, *** p ≤ 0.001.
2.2. CGA Induces Mitochondrial Reactive Oxygen Species (ROS) Production
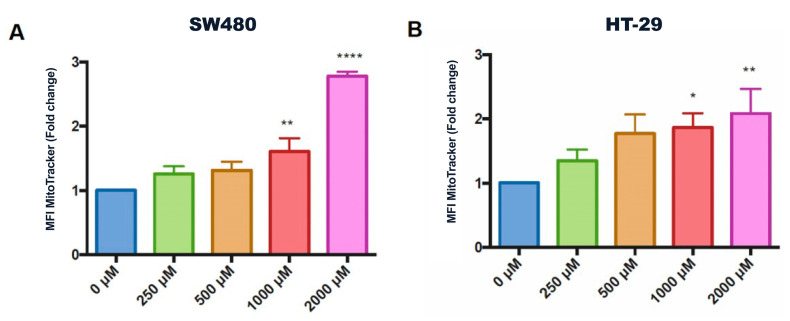
ROS production was assessed to investigate the mode of action of CGA on colon cancer cells. Figure 4 shows the quantification of the mean fluorescence intensity of MitoTracker Red CMXRos in SW480 and HT-29 cells, with a dose-dependent increase in ROS detection. SW480 cells showed higher ROS production after CGA treatment. Mitochondrial ROS increase can depolarize the mitochondrial membrane, as was observed in the previous results for SW480, which could result in increased activation of proapoptotic molecules, such as Caspase-3 [39].
Figure 4.
Effects of CGA on mitochondrial ROS production in colorectal cancer cells. (A) SW480 and (B) HT-29 cells were treated with CGA as indicated concentrations. MitoTracker Red CMXRos, a red fluorescent dye that stains mitochondria in live cells and fluoresces upon oxidation, was used to examine mitochondrial changes in ROS levels. One-way ANOVA for concentration effect, differences with respect to untreated cells, where * p ≤ 0.05, ** p ≤ 0.01, **** p ≤ 0.0001.
2.3. CGA Produces DNA Fragmentation Preferentially in SW480 Cells
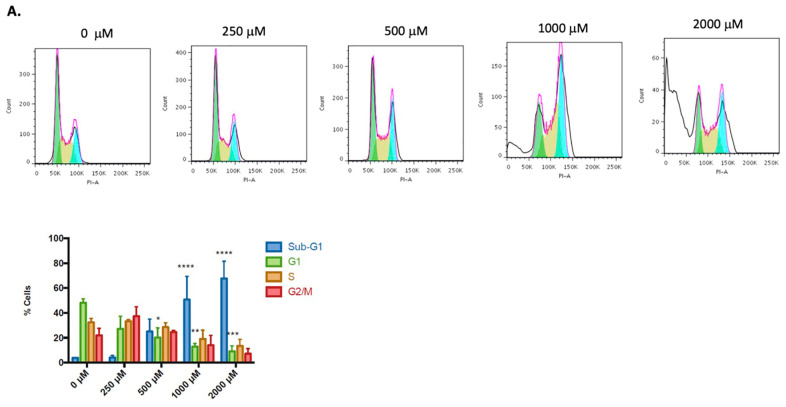
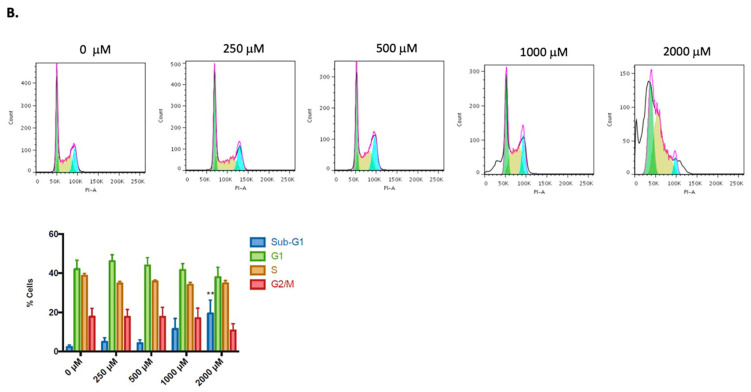
An analysis of the cell cycle distribution was used to describe the antiproliferative effect of CGA in colorectal cancer cells (Figure 5). Each cell line had a typical cell cycle distribution, with most cells in the G1 phase. At 500 μM and higher doses, CGA-treated cells showed a significant drop in the G1 phase percentage compared with untreated cells. This decrease in G1 cells occurred with an increased percentage of cells in the sub-G1 phase. The characteristic sub-G1 peak could be fragmented DNA marked with low-level DNA fluorescence, suggesting apoptotic cell death, as has been reported by other authors [40,41,42]. DNA fragmentation is more significant in SW480 (p < 0.001, Figure 5A) than in HT-29 cells (p < 0.01, Figure 5B).
Figure 5.
Cell cycle distribution after CGA treatment (24 h) in colorectal cancer cell lines SW480 (A) and HT-29 (B). The figure shows a representative histogram and bar graphs of flow cytometry analysis of the cell cycle distribution in the different phases. Two-way ANOVA for sub-G1, G1, S, and G2/M populations, displaying the difference with respect to untreated cells, where * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
2.4. Molecular Docking
Molecular docking studies were performed between CGA and two crucial proteins in the WNT/β-catenin pathway, and known inhibitors were used for validation. CGA showed a higher affinity for both sites of β-catenin than C2 and iCRT14 (Table 1) and a similar affinity for LRP6 E3 as niclosamide (Table 2). In the allosteric site of β-catenin, C2 showed an affinity of −4.6 kcal/mol, while CGA was −5.5 kcal/mol. Particularly, CGA presented more hydrogen bonds with the β-catenin sites than the validation compounds, as shown in Figure 6 and Figure 7.
Table 1.
Affinity and interactions of CGA and validation compounds (iCRT14 and C2) with β-catenin at the biding (US) and allosteric (AS) sites.
| Protein | Compound | Vina Score (Kcal/mol) | Protein-Ligand Interactions | ||
|---|---|---|---|---|---|
| Hydrogen Bonds | π Interactions | Hydrophobic Interactions | |||
| β-catenin AS | CGA | −5.5 | ARG587 | ||
| ASP583 | |||||
| HIS585 | |||||
| PRO521 | |||||
| HIS524 | |||||
| ALA525 | |||||
| GLN623 | |||||
| C2 | −4.6 | ARG587 | |||
| ASP583 | |||||
| VAL584 | |||||
| β-catenin US | CGA | −6.5 | ASN430 | HIS470 | ARG469 |
| LYS435 | |||||
| ARG474 | |||||
| ASN516 | |||||
| SER473 | |||||
| iCRT14 | −5.3 | ARG469 | ARG474 ARG515 |
||
| HIS470 | |||||
| LYS508 | |||||
Table 2.
Affinity and interactions of CGA and Niclosamide with LRP6.
| Protein | Compound | Vina Score (Kcal/mol) | Protein–Ligand Interactions | ||
|---|---|---|---|---|---|
| Hydrogen Bonds | π Interactions | Hydrophobic Interactions | |||
| LRP6 | CGA | −6.3 | SER665 GLU708 |
GLU708 | |
| ARG751 | |||||
| TRP767 | |||||
| LEU810 | |||||
| PHE836 | |||||
| Niclosamide | −6.3 | ASP811 HIS834 |
HIS834 | TRP767 | |
| LEU810 | |||||
| PHE836 | |||||
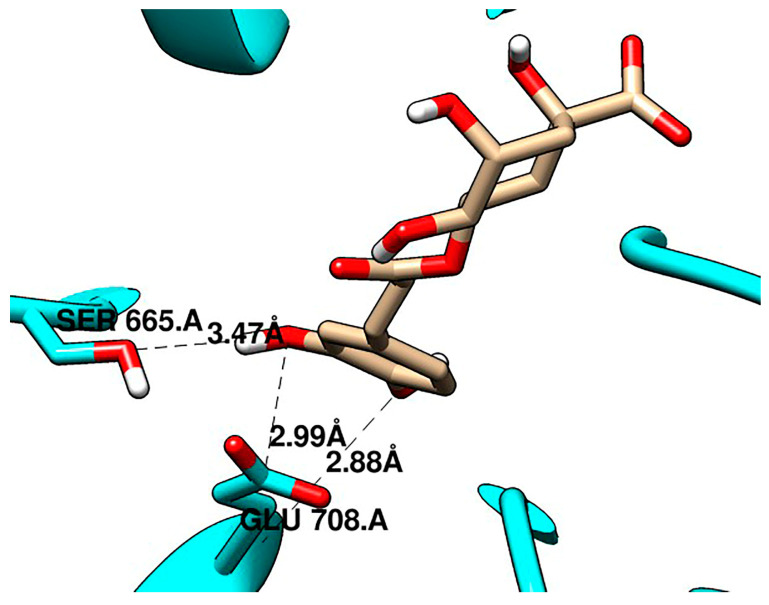
Figure 6.
CGA interacting with β-catenin (A) at the allosteric site and (B) at the binding site.
Figure 7.
CGA interacting with LRP6 at the E3 site.
3. Discussion
Although different studies have reported several biological properties of CGA [43,44], there still needs to be clear evidence about the role of this natural compound in CRC. In the present study, we showed that CGA induced ROS production and mitochondrial hyperpolarization, which results in the DNA fragmentation and reduced cell viability of colon cancer cells, which harbors alterations in the Wnt/β-catenin pathway. Additionally, we explore the interaction of CGA with β-catenin and LRP6 by in silico approaches to rationalize if the in vitro biological results could be potentially related to the possible modulation of the Wnt/β-catenin pathway.
According to our results, the concentrations of CGA required to achieve the desired biological effect in colorectal cancer cells are higher than the concentrations absorbed from food, and also in other reports that use CGA in lower doses [45,46]. However, generally, they do not determine the IC50 to select the working doses [11,14,17]. The advantage of using the concentrations suggested by the IC50 curves is that the sensitivity of every model is considered.
Limitations of CGA, such as low absorption and variable bioavailability, could affect scale-up to the in vivo study phase. Strategies to improve its pharmacokinetic profile could be a perspective of this work. For instance, some studies have shown that structural modifications of polyphenols, such as resveratrol and curcumin, increased their cytotoxic, antiproliferative, and proapoptotic effect [47,48]. Particularly, we have initiated a new project where we synthesize derivatives of CGA that showed a lower IC50 than CGA. However, it is still unknown if it could be due to a better pharmacokinetic profile or a higher derivative activity [49].
In this work, we observed an incremented ROS production in both the SW480 and the HT-29 cell lines (Figure 4) in the treatment with CGA, which results in mitochondrial hyperpolarization and an increase in sub-G1 cell population (Figure 5), suggesting DNA fragmentation (a hallmark of apoptotic cell death). Even though physiological levels of ROS play important roles in promoting normal cellular processes [50], ROS overproduction has been related to the oxidation of molecules, such as nucleic acids, lipids, carbohydrates, and proteins [51], which results in the alteration of the vital process, including cell-death-related mechanisms [52,53].
The results showed that CGA reduces the cell viability of the SW480 cells while not exhibiting a significant effect on the HT-29 cells (Figure 3); this behavior could be related to the enhancement in ROS production that could cause an alteration in the antioxidant balance of the cells. Although ROS production is observed at 1000 μM in both cell lines, the cell viability was not affected, probably because no damage was still involved in the macromolecules. Meanwhile, at 2000 μM, there is already a loss of cell homeostasis and induction of cell death [54].
Interestingly, different phytochemicals have been shown to possess pro-oxidant properties in the context of cancer through the induction of ROS accumulation in cancer cells and the subsequent activation of apoptotic cell death [51]. This is the case of complex mixtures of phytochemicals, such as Mangifera indica L. peel extracts [55], Passiflora edulis f. flavicarpa leaf extracts [56], Vaccinium meridionale Swartz juice [57], fermented nondigestible fraction from spent Coffea arabica grounds [58], fermented nondigestible fraction from Moringa oleifera leaves [59], Persea americana pulp extracts [60], and independent natural compounds, including quercetin [61], procyanidins [62], cannabidiol [63], resveratrol [64], curcumin [65], catechins [66], p-coumaric acid [67], kahweol [68], and CGA [6,69,70], among others.
To retain their malignant phenotypes, cancer cells contain higher levels of ROS and a different redox state from their normal counterparts. Because of this, cancer cells are more susceptible than normal cells to an increase in ROS generation brought on by different agents, including polyphenols [71]. Polyphenols have been shown to affect the redox status in varying ways, depending on the dose of polyphenols and the physiologic context of the interaction [72]. In cancer cells, a different mechanism of ROS induction by polyphenols has been explored, including pro-oxidant properties in systems containing redox-active metals [73,74,75], inhibition of endogenous antioxidants, and alteration in the electron transport chain [76,77]. In all cases, the mechanism through which polyphenols induce ROS production depends on concentration, structure, cell type, and experimental design.
The differences observed in the cell lines in response to CGA treatment, being SW480 more susceptible than HT-29 cells (Figure 3, Figure 4 and Figure 5), could be explained by its genetic and epigenetic backgrounds, which have a direct impact on the constitutive activation of signaling pathways involved in colon cancer onset and progression, such as TP53, KRAS, BRAF, Wnt/β-catenin, among others [78,79]. Both HT-29 and SW480 have microsatellite stable (MSS) phenotypes but differ in CpG island methylator phenotype (CIMP) and consensus molecular subtypes (CMS), being HT-29 CIMP+ and CMS3 (metabolic) with a moderately active Wnt/β-catenin pathway and, SW480 CIMP- and CMS4 (mesenchymal) with an upregulation of molecules involved in epithelial to mesenchymal transition and matrix remodeling [79,80]. Interestingly, both cell lines harbor truncating mutations in the APC gene that are tightly related to their CMS status but have a different impact on Wnt/β-catenin pathway activation. On the one hand, SW480 cells have a truncating mutation at residue 1338 in one allele of the APC gene, where the catenin inhibitory domain is located for β-catenin ubiquitination and degradation. It has been suggested as a requirement to drive high basal levels of Wnt/β-catenin pathway activity; on the other hand, HT-29 cells have a truncating mutation at residue 1555 of the APC gene, but retain the catenin inhibitory domain and the β-catenin binding sites (15RA–15RD and 20R1–20R3), which allows maintaining the regulatory effect of the APC protein over β-catenin in this cell line [32,33,34]. This is important, considering that APC mutations have been shown to cooperate with BRAF [81] and KRAS mutations [82,83] for the development of colon cancer, which are also genetic alterations present in HT-29 and SW480 cells, respectively [79]. Additionally, differences in the levels and activity of proteins of the detoxification machinery of the cells have been reported, including the ATP-binding cassette subfamily members ABCB1 and ABCG2, which are involved in the efflux of xenobiotics from cells, such as polyphenols [84], as well as the expression and activity of enzymes involved in the glucuronidation of free hydroxyl groups present in the chemical structures of polyphenolic compounds [85,86,87], increasing their polarity and water solubility for their excretion [88]. All these dissimilarities between SW480 and HT-29 could explain that despite ROS production being equivalent between both cell lines, the biological effect, including cell cycle distribution, presents significant differences.
Finally, to explore whether the in vitro biological results could be potentially related to Wnt/β-catenin pathway modulation, considering that the negative regulation of the pathway by natural products has been associated with the activation of cell-death-related mechanisms [89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108], molecular docking approaches were performed to analyze the interaction of CGA with crucial proteins in the Wnt/β-catenin pathway, named β-catenin and LRP6. In this manner, it was observed that CGA could interact with an allosteric site on the surface of β-catenin and with the binding surfaces of β-catenin involved in the interaction with the transcription factor TCF (Figure 6), and also with ectodomain 3 of LRP6 (Figure 7). It is important to note that all these interactions showed similar affinities, and in some cases even higher affinities, when compared with the validation compounds we used for the analysis (Table 1 and Table 2).
At the well-known β-catenin binding site, it has been reported that the residues Lys435 and Arg469 form a salt bridge. Additionally, it has been predicted that the compound UU-T01 binding to β-catenin around the residues Lys436, Arg469, and Lys508 and UU-T02 forms cation-π interactions with Arg474 and Arg515 [30]. Besides, docking studies predicted GB1874 binding to the β-catenin residues Arg469, Lys508, Asn426, Arg515, Arg474, and Arg435 [28]. Our study confirmed the salt bridge between Arg469 and CGA and the π interactions between Arg474 and Arg515 with iCRT14.
The binding pocket of DKK1 involves the Glu663, Ser665, Tyr706, Asp748, Lys770, Leu810, His834, Trp850, and Tyr875 residues of LRP6 as hot spot regions of this protein. Specifically, it formed hydrophobic bonds with Leu810, Asp811, Pro833, Tyr706, and Arg639, while it formed hydrogen bonds with Glu663, Glu708, Arg792, Asp811, Thr812, Asn813, Asp830, Leu832, and Arg1184 [24]. Our study observed the hydrophobic interactions between Leu810 from LRP6 with niclosamide and CGA, the hydrogen bonds between Glu708 and CGA, and Asp811 with niclosamide.
Previous studies have shown the potential of small molecule inhibitors, including C2 [31] and iCRT14 [29], and other molecules, to negatively regulate the Wnt/β-catenin pathway by the interaction with β-catenin and LRP6 proteins. Recently, the discovery of a small-molecule inhibitor (C2) has been reported. C2 targets an allosteric site on the surface of β-catenin in armadillo domain 8–10 and induces the proteasomal degradation of the protein. This results in the downregulation of Axin1, CyclinD1, and TCF4 proteins expression and the subsequent reduction in cell viability and tumor growth, even in the context of APC mutations [31]. Likewise, iCRT14 inhibits the direct interaction of β-catenin with TCF at the nucleus, interfering with its activity as a transcriptional activator, causing a downregulation of AXIN2, CCND1, and C-MYC gene expression and the inhibition of cell proliferation and invasion [29]. On the other hand, niclosamide suppressed the LRP6 expression in TNBC MDA-MB-231 cells and ER-positive breast cancer T-47D cells and inhibited breast cancer cell proliferation with IC50 values less than 1 mM [25]. Altogether, these observations support our hypothesis regarding the possible modulation of the Wnt/β-catenin pathway by CGA, even in the context of APC truncating mutations, considering the interaction of this molecule (Table 2) with β-catenin (Figure 6) and LRP6 (Figure 7), similarly as C2, iCRT14, and niclosamide. These findings are of great significance in the context of CRC, considering that APC mutations are an early and critical driver in the stepwise progression from adenoma to carcinoma [109], and also because the intestinal stem cell niche provides large amounts of Wnt ligands and amplifiers, which cooperates with intrinsic alterations of Wnt pathway members for the onset and progression of CRC [110].
As mentioned before, the Wnt/β-catenin pathway is implicated in the development of CRC [21]; hence improving targeted therapies against this pathway is mandatory for its translation to clinical practice [111,112,113]. In this regard, natural products showed the potential to modulate several signaling pathways involved in cancer development, including Wnt/β-catenin, through the downregulation of β-catenin and Wnt-target genes expression; the modulation of β-catenin phosphorylation, ubiquitination, and proteasomal degradation; and the inhibition of its nuclear translocation, among other mechanism [114,115], which results in the activation of cell-death-related mechanisms [89,90,91,92,93,94,95,96,97,99,100,104,108,115], supporting our hypothesis regarding the possible modulation of the Wnt/β-catenin pathway by CGA.
4. Materials and Methods
4.1. Materials
SW480 and HT-29 cells were commercially obtained from ATCC (Manassas, VA, USA). Cell culture reagents, including Dulbecco’s Modified Eagle Medium (DMEM), fetal bovine serum (FBS), antibiotics, and trypsin-EDTA, were purchased from Gibco (Grand Island, NY, USA). Chlorogenic acid was purchased from Sigma-Aldrich (St. Louis, MO, USA).
4.2. Cell Culture
The colon cancer cell lines SW480 and HT-29 were grown in DMEM supplemented with 5% FBS and RPMI media supplemented with 10% FBS, respectively. To complete the medium, 100 g/mL penicillin and 100 g/mL streptomycin were added. The cell cultures were kept at 37 °C in a humidified incubator with 5% CO2 and 95% air. Cells were evaluated regularly under a microscope for proper morphology and adhesion and were subcultured before the confluence.
4.3. Treatment Outline
Colon cancer cell lines were plated in 6-well plates at a concentration of 2.5 × 105 cells/mL. Cells were cultured under standard conditions, and after 24 h, to ensure adhesion and exponential growth, cells were treated for 24 h with doses of 250, 500, 1000, and 2000 µM of CGA [7]. Cells were trypsinized, pelleted, and analyzed by flow cytometry for different tests to evaluate the biological effect after treatments. Data reported included at least three separate experiments per treatment group.
4.4. Cell Viability
As a measure of cell viability, the cytoplasmic membrane’s integrity and mitochondrial membrane permeability changes were examined using propidium iodide (Sigma, P4170) and DiOC6 (Molecular Probes D273), respectively. Cells were pelleted and stained with 1 mg/mL PI and 50 nM DiOC6 and incubated for 30 min at room temperature to assess the incorporation of the dyes after CGA treatments. Flow cytometry was used to analyze 10,000 events with BD LSRFortessa. FlowJo was used to calculate the mean fluorescence intensity (MFI).
4.5. Mitochondrial ROS Production
Mitochondrial ROS production was evaluated to delimit the mechanism of cell death induction. Treated colon cancer cells were dyed with 3 μM MitoTracker Red (Invitrogen, M7512) and stored at room temperature for 20 min. After that, cells were washed twice in phosphate-buffered saline and analyzed by flow cytometry (BD LSRFortessa). The mean fluorescence intensity (MFI) of MitoTracker was calculated using FlowJo.
4.6. DNA Content
DNA content was analyzed to determine the cell distribution in cell cycle phases. CGA-treated cells were collected and centrifuged. Afterward, the cell pellet was fixed in 70% cold ethanol for 1 h. Permeabilized cells were incubated with 100 μg/mL of RNase (Sigma, R5000), labeled with 100 μg/mL of propidium iodide (Sigma, P4170), for 30 min and analyzed by flow cytometry (BD LSRFortessa). FlowJo was used to analyze the distribution of cell cycle phases.
4.7. Molecular Docking
4.7.1. Ligand Selection and Preparation
Chlorogenic acid anion and validation ligands (C2, iCRT14, and niclosamide) were built and prepared using GaussView 5 [116] and Gaussian 09 [117] with B3LYP-6-31++G** approximation. All ligands were prepared using AutoDock Tools (ADT) [118].
4.7.2. Protein Preparation
The structures of the studied proteins β-catenin (PDB code 2GL7) and LRP6 (PDB code 3S2K) were prepared using UCSF Chimera 8 [119] and ADT, and they were used without water molecules. Polar hydrogen atoms were automatically added to the protein, also AD4 type of atoms and Gasteiger charges.
Molecular docking was carried out using AutoDock Vina [120] with the parameters shown in Table 3. The pose with the best affinity for each site was chosen, and a visual inspection of the interactions was performed using a Discovery Studio Visualizer (BIOVIA), PLIP [121], and UCSF Chimera.
Table 3.
Docking parameters.
| Protein Subsite | Center of the Grid | Size | Exhaustiveness |
|---|---|---|---|
| β-catenin US | x = 11.527, y = 22.308, z = 62.347 | 17 Å3 | 20 |
| β-catenin AS | x = 2.805, y = 14.864, z = 79.543 | 17 Å3 | 20 |
| LRP6 E3 | x = 26.038, y = 5.167, z = −15.270 | 24 Å3 | 20 |
The parameters described in Table 3 were established according to the literature. β-catenin has two binding sites; the well-known union site (β-catenin US) is located in the most crucial interaction between the residue Asp16 from TCF4 and Lys435 and His470 of β-catenin [30]. For this reason, the NZ atom from Lys435 was chosen as the center of the grid for β-catenin US. On the other hand, an allosteric site of β-catenin (β-catenin AS) was recently reported, which included the residues Pro521, Arg528, and Asp583 [31], and the atom ND1 from His524, located near the center between the three reported residues, was chosen. On LRP6, the grid’s center was chosen from the coordinates of Gly227 from DKK1.
4.8. Statistical Analysis
ANOVA, followed by Fisher’s protected least significant difference (LSD) tests, was carried out to calculate statistical differences among nontreated cells and different doses of the treatments with CGA. p ≤ 0.05 was considered statistically significant. Data represent the results of a minimum of three independent experiments. Results are expressed as mean ± standard error of the mean (SEM). For graphs and analysis, the GraphPad Prism software was employed.
5. Conclusions
Despite the significant advances in the diagnosis of CRC, current treatments confer limited benefit, which makes this disease the third leading cause of cancer death. CRC development and progression involve altering regulatory mechanisms in one or more members of the Wnt/β-catenin signaling pathway. For this reason, identifying substances capable of modulating the Wnt/β-catenin signaling has been a significant effort for the scientific community. It is worth noting that some new compounds have recently been described as inhibitors of different components of this signaling pathway. Particular attention has been given to polyphenols. However, a long way still must be paved to achieve treatment success for CRC. CGA could be a potential coadjuvant in CRC therapy. In the present study, the induction of mitochondrial hyperpolarization, DNA fragmentation by CGA, and interactions between CGA with β-catenin and LRP6 suggest possible modulation of the Wnt/β-catenin pathway. Differences in sensitivity between SW480 and HT-29 would be related to the basal transcriptional activity of this signaling pathway in two lines. Our results provide an exciting starting point on the effect of CGA in the context of CRC and the possible modulation of the Wnt pathway.
Additional research is needed for a more in-depth understanding of this mechanism. For instance, future research regarding Wnt/β-catenin signaling in CRC should focus on (1) achieving a deeper understanding of crosstalk among the AKT/PI3K, NOTCH, mTOR, and Wnt/β-catenin pathways; (2) optimizing and evaluating other natural compounds as Wnt/β-catenin inhibitors while also being highly selective to avoid unnecessary side effects; (3) identifying additional inhibitors downstream of the Wnt/β-catenin signaling pathway; and (4) considering CGA structural modifications to improve the pharmacological profile and/or the affinity for β-catenin and LRP6. (5) In order to elucidate the relationship between ROS production and cell death in CGA-treated cells, other experiments should be performed. Treatments with antioxidants that block or mitigate ROS production would allow us to determine whether ROS is a mediator of this biological effect.
Author Contributions
The manuscript was written through contributions from all authors. Conceptualization, I.C.H.-C., G.A.S.-G., J.P.-D. and D.U.; formal analysis and investigation, L.C.V.-V., J.P.-D., G.A.S.-G. and I.C.H.-C.; writing—original draft preparation, L.C.V.-V., I.C.H.-C., G.A.S.-G., J.P.-D. and D.U.; writing—review and editing, I.C.H.-C., J. P-D.; supervision,. I.C.H.-C., J.P.-D.; project administration, J.P.-D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by MINCIENCIAS, grant number 115080763215 CT 811-2018; Instituto Tecnológico Metropolitano; and Universidad de Antioquia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization Cancer Today. [(accessed on 30 November 2022)]. Available online: https://gco.iarc.fr/
- 2.Cheng X., Xu X., Chen D., Zhao F., Wang W. Therapeutic Potential of Targeting the Wnt/β-Catenin Signaling Pathway in Colorectal Cancer. Biomed. Pharmacother. 2019;110:473–481. doi: 10.1016/j.biopha.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 3.Wild C.P., Weiderpass E.S.B., editors. World Cancer Report: Cancer Research for Cancer Prevention. International Agency for Research on Cancer; 2020. [(accessed on 23 January 2023)]. Available online: http://publications.iarc.fr/586. [Google Scholar]
- 4.Chen H.J., Hsu L.S., Shia Y.T., Lin M.W., Lin C.M. The β-Catenin/TCF Complex as a Novel Target of Resveratrol in the Wnt/β-Catenin Signaling Pathway. Biochem. Pharmacol. 2012;84:1143–1153. doi: 10.1016/j.bcp.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa S., Ohishi T., Miyoshi N., Oishi Y. Anti-Cancer Effects of Green Tea Epigallocatchin-3-Gallate and Coffee Chlorogenic Acid. Molecules. 2020;25:4553. doi: 10.3390/molecules25194553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ekbatan S.S., Li X.Q., Ghorbani M., Azadi B., Kubow S. Chlorogenic Acid and Its Microbial Metabolites Exert Anti-Proliferative Effects, S-Phase Cell-Cycle Arrest and Apoptosis in Human Colon Cancer Caco-2 Cells. Int. J. Mol. Sci. 2018;19:723. doi: 10.3390/ijms19030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villota H., Moreno-Ceballos M., Santa-González G.A., Uribe D., Castañeda I.C.H., Preciado L.M., Pedroza-Díaz J. Biological Impact of Phenolic Compounds from Coffee on Colorectal Cancer. Pharmaceuticals. 2021;14:761. doi: 10.3390/ph14080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villota H., Santa-González G.A., Uribe D., Henao I.C., Arroyave-Ospina J.C., Barrera-Causil C.J., Pedroza-Díaz J. Modulatory Effect of Chlorogenic Acid and Coffee Extracts on Wnt/β-Catenin Pathway in Colorectal Cancer Cells. Nutrients. 2022;14:4880. doi: 10.3390/nu14224880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan Y., Li J., Han J., Hou N., Song Y., Dong L. Chlorogenic Acid Enhances the Effects of 5-Fluorouracil in Human Hepatocellular Carcinoma Cells through the Inhibition of Extracellular Signal-Regulated Kinases. Anticancer. Drugs. 2015;26:540–546. doi: 10.1097/CAD.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Y., Liu N., Hou N., Dong L., Li J. Chlorogenic Acid Inhibits Hepatocellular Carcinoma in vitro and in vivo. J. Nutr. Biochem. 2017;46:68–73. doi: 10.1016/j.jnutbio.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y., Feng Y., Li Y., Hu Y., Zhang Q., Huang Y., Shi K., Ran C., Hou J., Zhou G., et al. Chlorogenic Acid Decreases Malignant Characteristics of Hepatocellular Carcinoma Cells by Inhibiting DNMT1 Expression. Front. Pharmacol. 2020;11:867. doi: 10.3389/fphar.2020.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng A., Liang X., Zhu S., Liu C., Wang S., Zhang Q., Zhao J., Song L. Chlorogenic Acid Induces Apoptosis, Inhibits Metastasis and Improves Antitumor Immunity in Breast Cancer via the NF-ΚB Signaling Pathway. Oncol. Rep. 2021;45:717–727. doi: 10.3892/or.2020.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster C., Wolpert N., Moustaid-Moussa N., Gollahon L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants. 2022;11:591. doi: 10.3390/antiox11030591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamagata K., Izawa Y., Onodera D., Tagami M. Chlorogenic Acid Regulates Apoptosis and Stem Cell Marker-Related Gene Expression in A549 Human Lung Cancer Cells. Mol. Cell. Biochem. 2018;441:9–19. doi: 10.1007/s11010-017-3171-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Du H., Chen P. Chlorogenic Acid Inhibits the Proliferation of Human Lung Cancer A549 Cell Lines by Targeting Annexin A2 in vitrso and in vivo. Biomed. Pharm. 2020;131:110673. doi: 10.1016/j.biopha.2020.110673. [DOI] [PubMed] [Google Scholar]
- 16.Yang J.S., Liu C.W., Ma Y.S., Weng S.W., Tang N.Y., Wu S.H., Ji B.C., Ma C.Y., Ko Y.C., Funayama S., et al. Chlorogenic Acid Induces Apoptotic Cell Death in U937 Leukemia Cells through Caspase-and Mitochondria-Dependent Pathways. In Vivo. 2012;26:971–978. [PubMed] [Google Scholar]
- 17.Liu Y.J., Zhou C.Y., Qiu C.H., Lu X.M., Wang Y.T. Chlorogenic Acid Induced Apoptosis and Inhibition of Proliferation in Human Acute Promyelocytic Leukemia HL-60 Cells. Mol. Med. Rep. 2013;8:1106–1110. doi: 10.3892/mmr.2013.1652. [DOI] [PubMed] [Google Scholar]
- 18.Sapio L., Salzillo A., Illiano M., Ragone A., Spina A., Chiosi E., Pacifico S., Catauro M., Naviglio S. Chlorogenic Acid Activates ERK1/2 and Inhibits Proliferation of Osteosarcoma Cells. J. Cell. Pharmacol. 2020;235:3741–3752. doi: 10.1002/jcp.29269. [DOI] [PubMed] [Google Scholar]
- 19.Wang X., Liu J., Xie Z., Rao J., Xu G., Huang K., Li W., Yin Z. Chlorogenic Acid Inhibits Proliferation and Induces Apoptosis in A498 Human Kidney Cancer Cells via Inactivating PI3K/Akt/MTOR Signalling Pathway. J. Pharm. Pharm. 2019;71:1100–1109. doi: 10.1111/jphp.13095. [DOI] [PubMed] [Google Scholar]
- 20.Disoma C., Zhou Y., Li S., Peng J., Xia Z. Wnt/β-Catenin Signaling in Colorectal Cancer: Is Therapeutic Targeting Even Possible? Biochimie. 2022;195:39–53. doi: 10.1016/j.biochi.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 21.Pai S.G., Carneiro B.A., Mota J.M., Costa R., Leite C.A., Barroso-Sousa R., Kaplan J.B., Chae Y.K., Giles F.J. Wnt/Beta-Catenin Pathway: Modulating Anticancer Immune Response. J. Hematol. Oncol. 2017;10:101. doi: 10.1186/s13045-017-0471-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg A., Kant K., Roy K.K., Sahoo A., Malakar C.C., Gupta S. Docking-Based Evaluation against Human Tankyrase-1 and Tankyrase-2 Enzyme. Mater. Today Proc. 2022;57:300–306. doi: 10.1016/j.matpr.2022.03.095. [DOI] [Google Scholar]
- 23.Enayatkhani M., Salimi M., Azadmanesh K., Teimoori-Toolabi L. In-Silico Identification of New Inhibitors for Low-Density Lipoprotein Receptor-Related Protein6 (LRP6) J. Biomol. Struct. Dyn. 2022;40:4440–4450. doi: 10.1080/07391102.2020.1857843. [DOI] [PubMed] [Google Scholar]
- 24.Rismani E., Rahimi H., Arab S.S., Azadmanesh K., Karimipoor M., Teimoori-Toolabi L. Computationally Design of Inhibitory Peptides against Wnt Signaling Pathway: In Silico Insight on Complex of DKK1 and LRP6. Int. J. Pept. Res. Ther. 2018;24:49–60. doi: 10.1007/s10989-017-9589-1. [DOI] [Google Scholar]
- 25.Lu W., Lin C., Roberts M.J., Waud W.R., Piazza G.A., Li Y. Niclosamide Suppresses Cancer Cell Growth by Inducing Wnt Co-Receptor LRP6 Degradation and Inhibiting the Wnt/β-Catenin Pathway. PLoS ONE. 2011;6:e29290. doi: 10.1371/journal.pone.0029290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suliman M.A., Zhang Z., Na H., Ribeiro A.L.L., Zhang Y., Niang B., Hamid A.S., Zhang H., Xu L., Zuo Y. Niclosamide Inhibits Colon Cancer Progression through Downregulation of the Notch Pathway and Upregulation of the Tumor Suppressor MiR-200 Family. Int. J. Mol. Med. 2016;38:776–784. doi: 10.3892/ijmm.2016.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang F., Ye T., Liu Z., Fang A., Luo Y., Wei W., Li Y., Li Y., Zeng A., Deng Y., et al. Niclosamide Induces Colorectal Cancer Apoptosis, Impairs Metastasis and Reduces Immunosuppressive Cells in vivo. RSC Adv. 2016;6:106019–106030. doi: 10.1039/C6RA21533E. [DOI] [Google Scholar]
- 28.Low J.L., Du W., Gocha T., Oguz G., Zhang X., Chen M.W., Masirevic S., Yim D.G.R., Tan I.B.H., Ramasamy A., et al. Molecular Docking-Aided Identification of Small Molecule Inhibitors Targeting β-Catenin-TCF4 Interaction. iScience. 2021;24:102544. doi: 10.1016/j.isci.2021.102544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonsalves F.C., Klein K., Carson B.B., Katz S., Ekas L.A., Evans S., Nagourney R., Cardozo T., Brown A.M.C., Das Gupta R. An RNAi-Based Chemical Genetic Screen Identifies Three Small-Molecule Inhibitors of the Wnt/Wingless Signaling Pathway. Proc. Natl. Acad. Sci. USA. 2011;108:5954–5963. doi: 10.1073/pnas.1017496108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koelman E.M.R., Yeste-Vázquez A., Grossmann T.N. Targeting the Interaction of β-Catenin and TCF/LEF Transcription Factors to Inhibit Oncogenic Wnt Signaling. Bioorg. Med. Chem. 2022;70:116920. doi: 10.1016/j.bmc.2022.116920. [DOI] [PubMed] [Google Scholar]
- 31.Cheltsov A., Nomura N., Yenugonda V.M., Roper J., Mukthavaram R., Jiang P., Her N.G., Babic I., Kesari S., Nurmemmedov E. Allosteric Inhibitor of β-Catenin Selectively Targets Oncogenic Wnt Signaling in Colon Cancer. Sci. Rep. 2020;10:8096. doi: 10.1038/s41598-020-60784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang J., Zhang W., Evans P.M., Chen X., He X., Liu C. Adenomatous Polyposis Coli (APC) Differentially Regulates β-Catenin Phosphorylation and Ubiquitination in Colon Cancer Cells. J. Biol. Chem. 2006;281:17751–17757. doi: 10.1074/jbc.M600831200. [DOI] [PubMed] [Google Scholar]
- 33.Chandra S.H.V., Wacker I., Appelt U.K., Behrens J., Schneikert J. A Common Role for Various Human Truncated Adenomatous Polyposis Coli Isoforms in the Control of Beta-Catenin Activity and Cell Proliferation. PLoS ONE. 2012;7:e34479. doi: 10.1371/journal.pone.0034479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Novellasdemunt L., Foglizzo V., Cuadrado L., Antas P., Kucharska A., Encheva V., Snijders A.P., Li V.S.W. USP7 Is a Tumor-Specific WNT Activator for APC-Mutated Colorectal Cancer by Mediating β-Catenin Deubiquitination. Cell Rep. 2017;21:612–627. doi: 10.1016/j.celrep.2017.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajabi S., Maresca M., Yumashev A.V., Choopani R., Hajimehdipoor H. The Most Competent Plant-derived Natural Products for Targeting Apoptosis in Cancer Therapy. Biomolecules. 2021;11:534. doi: 10.3390/biom11040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe S.W., Lin A.W. Apoptosis in Cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 37.Hu X., Wang L., He Y., Wei M., Yan H., Zhu H. Chlorogenic Acid Promotes Osteogenic Differentiation of Human Dental Pulp Stem Cells through Wnt Signaling. Stem. Cells Dev. 2021;30:641–650. doi: 10.1089/scd.2020.0193. [DOI] [PubMed] [Google Scholar]
- 38.Liu M., Qin J., Cong J., Yang Y. Chlorogenic Acids Inhibit Adipogenesis: Implications of Wnt/ β-Catenin Signaling Pathway. Int. J. Endocrinol. 2021;2021:2215274. doi: 10.1155/2021/2215274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao S., Chinkwo K., Santhakumar A., Johnson S., Blanchard C. Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts. Molecules. 2019;24:2465. doi: 10.3390/molecules24132465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plesca D., Mazumder S., Almasan A. Methods in Enzymology. Volume 446. Academic Press; Cambridge, MA, USA: 2008. Chapter Six: DNA Damage Response and Apoptosis; pp. 107–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kajstura M., Halicka H.D., Pryjma J., Darzynkiewicz Z. Discontinuous Fragmentation of Nuclear DNA during Apoptosis Revealed by Discrete “Sub-G1” Peaks on DNA Content Histograms. Cytom. Part A. 2007;71:125–131. doi: 10.1002/cyto.a.20357. [DOI] [PubMed] [Google Scholar]
- 42.Nicoletti I., Migliorati G., Pagliacci M.C., Grignani F., Riccardi C. A Rapid and Simple Method for Measuring Thymocyte Apoptosis by Propidium Iodide Staining and Flow Cytometry. J. Immunol. Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-O. [DOI] [PubMed] [Google Scholar]
- 43.Moreno-Ceballos M., Arroyave J.C., Cortes-Mancera F.M., Röthlisberger S. Chemopreventive Effect of Coffee against Colorectal Cancer and Hepatocellular Carcinoma. Int. J. Food Prop. 2019;22:536–555. doi: 10.1080/10942912.2019.1593193. [DOI] [Google Scholar]
- 44.Yepes Y., Uribe D., Röthlisberger S. A Review of the Chemopreventive Effects of the Main Bioactive Compounds in Coffee in Colorectal Cancer. J. Appl. Pharm. Sci. 2021;11:046–054. doi: 10.7324/JAPS.2021.110704. [DOI] [Google Scholar]
- 45.Ardini M.N., Irillo E.C., Atella F.N., Caccini C.S. Absorption of Phenolic Acids in Humans after Coffee Consumption. J. Agric. Food Chem. 2002;50:5735–5741. doi: 10.1021/jf0257547. [DOI] [PubMed] [Google Scholar]
- 46.Olthof M.R., Hollman P.C.H., Katan M.B. Human Nutrition and Metabolism Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans 1. J. Nutr. 2001;131:66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 47.Lee Y.Q., Rajadurai P., Abas F., Othman I., Naidu R. Proteomic Analysis on Anti-Proliferative and Apoptosis Effects of Curcumin Analog, 1,5-Bis(4-Hydroxy-3-Methyoxyphenyl)-1,4-Pentadiene-3-One-Treated Human Glioblastoma and Neuroblastoma Cells. Front. Mol. Biosci. 2021;8:645856. doi: 10.3389/fmolb.2021.645856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian B., Liu J. Resveratrol: A Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 2020;100:1392–1404. doi: 10.1002/jsfa.10152. [DOI] [PubMed] [Google Scholar]
- 49.ASSOCIAÇÃO BRASILEIRA DE QUÍMICA 35° CLAQ—Congresso Latinoamericano de Química 61° CBQ—Congresso Brasileiro de Química. [(accessed on 13 December 2022)]. Available online: https://www.abq.org.br/cbq/2022/trabalhos/11/875-762.html.
- 50.Sena L.A., Chandel N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NavaneethaKrishnan S., Rosales J.L., Lee K.Y. ROS-Mediated Cancer Cell Killing through Dietary Phytochemicals. Oxid. Med. Cell. Longev. 2019;2019:9051542. doi: 10.1155/2019/9051542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davalli P., Marverti G., Lauriola A., D’Arca D. Targeting Oxidatively Induced DNA Damage Response in Cancer: Opportunities for Novel Cancer Therapies. Oxid. Med. Cell. Longev. 2018;2018:2389523. doi: 10.1155/2018/2389523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Houten B., Santa-Gonzalez G.A., Camargo M. DNA Repair after Oxidative Stress: Current Challenges. Curr. Opin. Toxicol. 2018;7:9–16. doi: 10.1016/j.cotox.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santa-Gonzalez G.A., Gomez-Molina A., Arcos-Burgos M., Meyer J.N., Camargo M. Distinctive Adaptive Response to Repeated Exposure to Hydrogen Peroxide Associated with Upregulation of DNA Repair Genes and Cell Cycle Arrest. Redox Biol. 2016;9:124–133. doi: 10.1016/j.redox.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lauricella M., Galbo V.L., Cernigliaro C., Maggio A., Piccionello A.P., Calvaruso G., Carlisi D., Emanuele S., Giuliano M., D’Anneo A. The Anti-Cancer Effect of Mangifera indica, L. Peel Extract Is Associated to ΓH2Ax-Mediated Apoptosis in Colon Cancer Cells. Antioxidants. 2019;8:422. doi: 10.3390/antiox8100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramirez V., Arango S.S., Maldonado M.E., Uribe D., Aguillon J., Quintero J.P., Loango N. Biological Activity of Passiflora edulis f. Flavicarpa Ethanolic Leaves Extract on Human Colonic Adenocarcinoma Cells. J. Appl. Pharm. Sci. 2019;9:64–71. doi: 10.7324/JAPS.2019.90209. [DOI] [Google Scholar]
- 57.Agudelo C.D., Arango S., Cortés-Mancera F., Rojano B., Maldonado M.E. Antiproliferative and Pro-Apoptotic Effects of Andean Berry Juice (Vaccinium Meridionale Swartz) on Human Colon Adenocarcinoma SW480 Cells. J. Med. Plants Res. 2017;11:393–402. doi: 10.5897/jmpr2017.6401. [DOI] [Google Scholar]
- 58.García-Gutiérrez N., Maldonado-Celis M.E., Rojas-López M., Loarca-Piña G.F., Campos-Vega R. The Fermented Non-Digestible Fraction of Spent Coffee Grounds Induces Apoptosis in Human Colon Cancer Cells (SW480) J. Funct. Foods. 2017;30:237–246. doi: 10.1016/j.jff.2017.01.014. [DOI] [Google Scholar]
- 59.Caicedo-Lopez L.H., Cuellar-Nuñez M.L., Luzardo-Ocampo I., Campos-Vega R., Lóarca-Piña G. Colonic Metabolites from Digested Moringa Oleifera Leaves Induced HT-29 Cell Death via Apoptosis, Necrosis, and Autophagy. Int. J. Food Sci. Nutr. 2021;72:485–498. doi: 10.1080/09637486.2020.1849039. [DOI] [PubMed] [Google Scholar]
- 60.Ding H., Han C., Guo D., Chin Y.W., Ding Y., Kinghorn A.D., D’Ambrosio S.M. Selective Induction of Apoptosis of Human Oral Cancer Cell Lines by Avocado Extracts via a ROS-Mediated Mechanism. Nutr. Cancer. 2009;61:348–356. doi: 10.1080/01635580802567158. [DOI] [PubMed] [Google Scholar]
- 61.Raja S.B., Rajendiran V., Kasinathan N.K., Amrithalakshmi A.P., Venkatabalasubramanian S., Murali M.R., Devaraj H., Devaraj S.N. Differential Cytotoxic Activity of Quercetin on Colonic Cancer Cells Depends on ROS Generation through COX-2 Expression. Food Chem. Toxicol. 2017;106:92–106. doi: 10.1016/j.fct.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Maldonado-Celis M.E., Bousserouel S., Gossé F., Minker C., Lobstein A., Raul F. Differential Induction of Apoptosis by Apple Procyanidins in TRAIL-Sensitive Human Colon Tumor Cells and Derived TRAIL-Resistant Metastatic Cells. J. Cancer Mol. 2009;5:21–30. [Google Scholar]
- 63.Zhang X., Qin Y., Pan Z., Li M., Liu X., Chen X., Qu G., Zhou L., Xu M., Zheng Q., et al. Cannabidiol Induces Cell Cycle Arrest and Cell Apoptosis in Human Gastric Cancer SGC-7901 Cells. Biomolecules. 2019;9:302. doi: 10.3390/biom9080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heo J.R., Kim S.M., Hwang K.A., Kang J.H., Choi K.C. Resveratrol Induced Reactive Oxygen Species and Endoplasmic Reticulum Stress-Mediated Apoptosis, and Cell Cycle Arrest in the A375SM Malignant Melanoma Cell Line. Int. J. Mol. Med. 2018;42:1427–1435. doi: 10.3892/ijmm.2018.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sritharan S., Sivalingam N. Curcumin Induced Apoptosis Is Mediated through Oxidative Stress in Mutated P53 and Wild Type P53 Colon Adenocarcinoma Cell Lines. J. Biochem. Mol. Toxicol. 2021;35:e22616. doi: 10.1002/jbt.22616. [DOI] [PubMed] [Google Scholar]
- 66.Khiewkamrop P., Phunsomboon P., Richert L., Pekthong D., Srisawang P. Epistructured Catechins, EGCG and EC Facilitate Apoptosis Induction through Targeting de Novo Lipogenesis Pathway in HepG2 Cells. Cancer Cell Int. 2018;18:46. doi: 10.1186/s12935-018-0539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaganathan S.K., Supriyanto E., Mandal M. Events Associated with Apoptotic Effect of p-Coumaric Acid in HCT-15 Colon Cancer Cells. World, J. Gastroenterol. 2013;19:7726–7734. doi: 10.3748/wjg.v19.i43.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cárdenas C., Quesada A.R., Medina M.Á. Insights on the Antitumor Effects of Kahweol on Human Breast Cancer: Decreased Survival and Increased Production of Reactive Oxygen Species and Cytotoxicity. Biochem. Biophys. Res. Commun. 2014;447:452–458. doi: 10.1016/j.bbrc.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 69.Hou N., Liu N., Han J., Yan Y., Li J. Chlorogenic Acid Induces Reactive Oxygen Species Generation and Inhibits the Viability of Human Colon Cancer Cells. Anticancer. Drugs. 2017;28:59–65. doi: 10.1097/CAD.0000000000000430. [DOI] [PubMed] [Google Scholar]
- 70.Murad L.D., Soares N.D.C.P., Brand C., Monteiro M.C., Teodoro A.J. Effects of Caffeic and 5-Caffeoylquinic Acids on Cell Viability and Cellular Uptake in Human Colon Adenocarcinoma Cells. Nutr. Cancer. 2015;67:532–542. doi: 10.1080/01635581.2015.1004736. [DOI] [PubMed] [Google Scholar]
- 71.Gibellini L., Pinti M., Nasi M., de Biasi S., Roat E., Bertoncelli L., Cossarizza A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers. 2010;2:1288–1311. doi: 10.3390/cancers2021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forester S.C., Lambert J.D. The Role of Antioxidant versus Pro-Oxidant Effects of Green Tea Polyphenols in Cancer Prevention. Mol. Nutr. Food Res. 2011;55:844–854. doi: 10.1002/mnfr.201000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakihama Y., Cohen M.F., Grace S.C., Yamasaki H. Plant Phenolic Antioxidant and Prooxidant Activities: Phenolics-Induced Oxidative Damage Mediated by Metals in Plants. Toxicology. 2002;177:67–80. doi: 10.1016/S0300-483X(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 74.Zhu N., Huang T.C., Yu Y., LaVoie E.J., Yang C.S., Ho C.T. Identification of Oxidation Products of (-)-Epigallocatechin Gallate and (-)-Epigallocatechin with H2O2. J. Agric. Food Chem. 2000;48:979–981. doi: 10.1021/jf991188c. [DOI] [PubMed] [Google Scholar]
- 75.Malik A., Azam S., Hadi N., Hadi S.M. DNA Degradation by Water Extract of Green Tea in the Presence of Copper Ions: Implications for Anticancer Properties. Phyther. Res. 2003;17:358–363. doi: 10.1002/ptr.1149. [DOI] [PubMed] [Google Scholar]
- 76.Catalán M., Olmedo I., Faúndez J., Jara J.A. Medicinal Chemistry Targeting Mitochondria: From New Vehicles and Pharmacophore Groups to Old Drugs with Mitochondrial Activity. Int. J. Mol. Sci. 2020;21:8684. doi: 10.3390/ijms21228684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.del Blanquer-Rosselló M.M., Hernández-López R., Roca P., Oliver J., Valle A. Resveratrol Induces Mitochondrial Respiration and Apoptosis in SW620 Colon Cancer Cells. Biochim. Biophys. Acta-Gen. Subj. 2017;1861:431–440. doi: 10.1016/j.bbagen.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Ahmed D., Eide P.W., Eilertsen I.A., Danielsen S.A., Eknæs M., Hektoen M., Lind G.E., Lothe R.A. Epigenetic and Genetic Features of 24 Colon Cancer Cell Lines. Oncogenesis. 2013;2:e71. doi: 10.1038/oncsis.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berg K.C.G., Eide P.W., Eilertsen I.A., Johannessen B., Bruun J., Danielsen S.A., Bjørnslett M., Meza-Zepeda L.A., Eknæs M., Lind G.E., et al. Multi-Omics of 34 Colorectal Cancer Cell Lines—A Resource for Biomedical Studies. Mol. Cancer. 2017;16:116. doi: 10.1186/s12943-017-0691-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh M.P., Rai S., Pandey A., Singh N.K., Srivastava S. Molecular Subtypes of Colorectal Cancer: An Emerging Therapeutic Opportunity for Personalized Medicine. Genes Dis. 2021;8:133–145. doi: 10.1016/j.gendis.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fennell L.J., Kane A., Liu C., McKeone D., Fernando W., Su C., Bond C., Jamieson S., Dumenil T., Patch A.M., et al. APC Mutation Marks an Aggressive Subtype of BRAF Mutant Colorectal Cancers. Cancers. 2020;12:1171. doi: 10.3390/cancers12051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luo F., Poulogiannis G., Ye H., Hamoudi R., Arends M.J. Synergism between K-RasVal12 and Mutant Apc Accelerates Murine Large Intestinal Tumourigenesis. Oncol. Rep. 2011;26:125–133. doi: 10.3892/or.2011.1288. [DOI] [PubMed] [Google Scholar]
- 83.Kim J.H., Park J.M., Roh Y.J., Kim I.W., Hasan T., Choi M.G. Enhanced Efficacy of Photodynamic Therapy by Inhibiting ABCG2 in Colon Cancers. BMC Cancer. 2015;15:504. doi: 10.1186/s12885-015-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aires V., Colin D.J., Doreau A., Pietro A.D., Heydel J.M., Artur Y., Latruffe N., Delmas D. P-Glycoprotein 1 Affects Chemoactivities of Resveratrol against Human Colorectal Cancer Cells. Nutrients. 2019;11:7–9. doi: 10.3390/nu11092098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu M., Wang Q., Liu F., Cheng X., Wu X., Wang H., Wu M., Ma Y., Wang G., Hao H. UDP-Glucuronosyltransferase 1A Compromises Intracellular Accumulation and Anti-Cancer Effect of Tanshinone IIA in Human Colon Cancer Cells. PLoS ONE. 2013;8:e79172. doi: 10.1371/journal.pone.0079172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Landmann H., Proia D.A., He S., Ogawa L.S., Kramer F., Beibarth T., Grade M., Gaedcke J., Ghadimi M., Moll U., et al. UDP Glucuronosyltransferase 1A Expression Levels Determine the Response of Colorectal Cancer Cells to the Heat Shock Protein 90 Inhibitor Ganetespib. Cell Death Dis. 2014;5:e1411. doi: 10.1038/cddis.2014.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu H., Tian Z., Cui Y., Liu Z., Ma X. Chlorogenic Acid: A Comprehensive Review of the Dietary Sources, Processing Effects, Bioavailability, Beneficial Properties, Mechanisms of Action, and Future Directions. Compr. Rev. Food Sci. Food Saf. 2020;19:3130–3158. doi: 10.1111/1541-4337.12620. [DOI] [PubMed] [Google Scholar]
- 88.Wu B., Kulkarni K., Basu S., Zhang S., Hu M. First Pass Metabolism via UDP-Glucuronosyltransferase: A Barrier to Oral Bioavailability of Phenolics. Pharm. Sci. 2011;100:3655–3681. doi: 10.1002/jps.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen Y., Liu Z.H., Xia J., Li X.P., Li K.Q., Xiong W., Li J., Chen D.L. 20(S)-Ginsenoside Rh2 Inhibits the Proliferation and Induces the Apoptosis of KG-1a Cells through the Wnt/β-Catenin Signaling Pathway. Oncol. Rep. 2016;36:137–146. doi: 10.3892/or.2016.4774. [DOI] [PubMed] [Google Scholar]
- 90.Ye Z.N., Yuan F., Liu J.Q., Peng X.R., An T., Li X., Kong L.M., Qiu M.H., Li Y. Physalis Peruviana-Derived 4β-Hydroxywithanolide E, a Novel Antagonist of Wnt Signaling, Inhibits Colorectal Cancer in vitro and in vivo. Molecules. 2019;24:1146. doi: 10.3390/molecules24061146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ravichandran K., Velmurugan B., Gu M., Singh R.P., Aagarwal R. Inhibitory Effect of Silibinin against Azoxymethane-Induced Colon Tumorigenesis in A/J Mice. Bone. 2010;16:4595–4606. doi: 10.1158/1078-0432.CCR-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li H., Huang J., Yang B., Xiang T., Yin X., Peng W., Cheng W., Wan J., Luo F., Li H., et al. Mangiferin Exerts Antitumor Activity in Breast Cancer Cells by Regulating Matrix Metalloproteinases, Epithelial to Mesenchymal Transition, and β-Catenin Signaling Pathway. Toxicol. Appl. Pharmacol. 2013;272:180–190. doi: 10.1016/j.taap.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 93.Prasad C.P., Rath G., Mathur S., Bhatnagar D., Ralhan R. Potent Growth Suppressive Activity of Curcumin in Human Breast Cancer Cells: Modulation of Wnt/β-Catenin Signaling. Chem. Biol. Interact. 2009;181:263–271. doi: 10.1016/j.cbi.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 94.Sultan A.S., Khalil M.I.M., Sami B.M., Alkhuriji A.F., Sadek O. Quercetin Induces Apoptosis in Triple-Negative Breast Cancer Cells via Inhibiting Fatty Acid Synthase and ß-Catenin. Int. J. Clin. Exp. Pathol. 2017;10:156–172. [Google Scholar]
- 95.Mineda A., Nishimura M., Kagawa T., Takiguchi E., Kawakita T., Abe A., Irahara M. Resveratrol Suppresses Proliferation and Induces Apoptosis of Uterine Sarcoma Cells by Inhibiting the Wnt Signaling Pathway. Exp. Ther. Med. 2019;17:2242–2246. doi: 10.3892/etm.2019.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang L., Zhu H., Yang X., Xie F., Peng J., Jiang D., Xie J., Qi M., Yu L. Shizukaol D, a Dimeric Sesquiterpene Isolated from Chloranthus Serratus, Represses the Growth of Human Liver Cancer Cells by Modulating Wnt Signalling Pathway. PLoS ONE. 2016;11:e0152012. doi: 10.1371/journal.pone.0152012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kaur M., Velmurugan B., Tyagi A., Agarwal C., Singh R.P., Agarwal R. Silibinin Suppresses Growth of Human Colorectal Carcinoma SW480 Cells in Culture and Xenograft through Down-Regulation of β-Catenin-Dependent Signaling. Neoplasia. 2010;12:415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ren K., Zhang W., Wu G., Ren J., Lu H., Li Z., Han X. Synergistic Anti-Cancer Effects of Galangin and Berberine through Apoptosis Induction and Proliferation Inhibition in Oesophageal Carcinoma Cells. Biomed. Pharmacother. 2016;84:1748–1759. doi: 10.1016/j.biopha.2016.10.111. [DOI] [PubMed] [Google Scholar]
- 99.Sur S., Pal D., Mandal S., Roy A., Panda C.K. Tea Polyphenols Epigallocatechin Gallete and Theaflavin Restrict Mouse Liver Carcinogenesis through Modulation of Self-Renewal Wnt and Hedgehog Pathways. J. Nutr. Biochem. 2016;27:32–42. doi: 10.1016/j.jnutbio.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 100.Xie C., Li X., Geng S., Wu J., Zhong C., Li X., Li Y., Chen Y., Wang X., Meng Y., et al. Wnt/β-Catenin Pathway Mediates (−)-Epigallocatechin-3-Gallate (EGCG) Inhibition of Lung Cancer Stem Cells. Biochem. Biophys. Res. Commun. 2017;482:15–21. doi: 10.1016/j.bbrc.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 101.Bi X., Zhao Y., Fang W., Yang W. Anticancer Activity of Panax Notoginseng Extract 20(S)-25-OCH3-PPD: Targetting β-Catenin Signalling. Clin. Exp. Pharmacol. Physiol. 2009;36:1074–1078. doi: 10.1111/j.1440-1681.2009.05203.x. [DOI] [PubMed] [Google Scholar]
- 102.Leow P.C., Tian Q., Ong Z.Y., Yang Z., Ee P.L.R. Antitumor Activity of Natural Compounds, Curcumin and PKF118-310, as Wnt/β-Catenin Antagonists against Human Osteosarcoma Cells. Investig. New Drugs. 2010;28:766–782. doi: 10.1007/s10637-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 103.Jia Y., Chen L., Guo S., Li Y. Baicalin Induced Colon Cancer Cells Apoptosis through MiR-217/DKK1-Mediated Inhibition of Wnt Signaling Pathway. Mol. Biol. Rep. 2019;46:1693–1700. doi: 10.1007/s11033-019-04618-9. [DOI] [PubMed] [Google Scholar]
- 104.Chen Y., Wang X.Q., Zhang Q., Zhu J.Y., Li Y., Xie C.F., Li X.T., Wu J.S., Geng S.S., Zhong C.Y., et al. (-)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients. 2017;9:572. doi: 10.3390/nu9060572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suh Y., Afaq F., Johnson J.J., Mukhtar H. A Plant Flavonoid Fisetin Induces Apoptosis in Colon Cancer Cells by Inhibition of COX2 and Wnt/EGFR/NF-ΚB-Signaling Pathways. Carcinogenesis. 2009;30:300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang Y., Chen H. Genistein Attenuates WNT Signaling by Up-Regulating SFRP2 in a Human Colon Cancer Cell Line. Exp. Biol. Med. 2011;236:714–722. doi: 10.1258/ebm.2011.010347. [DOI] [PubMed] [Google Scholar]
- 107.Hirata H., Ueno K., Nakajima K., Tabatabai Z.L., Hinoda Y., Ishii N., Dahiya R. Genistein Downregulates Onco-MiR-1260b and Inhibits Wnt-Signalling in Renal Cancer Cells. Br. J. Cancer. 2013;108:2070–2078. doi: 10.1038/bjc.2013.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang L., Tu Y., He W., Peng Y., Qiu Z. A Novel Mechanism of Hepatocellular Carcinoma Cell Apoptosis Induced by Lupeol via Brain-Derived Neurotrophic Factor Inhibition and Glycogen Synthase Kinase 3 Beta Reactivation. Eur. J. Pharmacol. 2015;762:55–62. doi: 10.1016/j.ejphar.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 109.Bugter J.M., Fenderico N., Maurice M.M. Mutations and Mechanisms of WNT Pathway Tumour Suppressors in Cancer. Nat. Rev. Cancer. 2021;21:5–21. doi: 10.1038/s41568-020-00307-z. [DOI] [PubMed] [Google Scholar]
- 110.van Neerven S.M., Vermeulen L. The Interplay between Intrinsic and Extrinsic Wnt Signaling in Controlling Intestinal Transformation. Differentiation. 2019;108:17–23. doi: 10.1016/j.diff.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jung Y.S., Park J.I. Wnt Signaling in Cancer: Therapeutic Targeting of Wnt Signaling beyond β-Catenin and the Destruction Complex. Exp. Mol. Med. 2020;52:183–191. doi: 10.1038/s12276-020-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang Y., Wang X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J. Hematol. Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ji Y., Lv J., Sun D., Huang Y. Therapeutic Strategies Targeting Wnt/β-Catenin Signaling for Colorectal Cancer (Review) Int. J. Mol. Med. 2021;49:1–17. doi: 10.3892/ijmm.2021.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sferrazza G., Corti M., Brusotti G., Pierimarchi P., Temporini C., Serafino A., Calleri E. Nature-Derived Compounds Modulating Wnt/β-Catenin Pathway: A Preventive and Therapeutic Opportunity in Neoplastic Diseases. Acta Pharm. Sin. B. 2020;10:1814–1834. doi: 10.1016/j.apsb.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yu W.K., Xu Z.Y., Yuan L., Mo S., Xu B., Cheng X.D., Qin J.J. Targeting β-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharmacol. 2020;11:984. doi: 10.3389/fphar.2020.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nielsen A., Holder A. GaussView Version 5.0. [(accessed on 23 January 2023)]. Available online: https://gaussian.com/gaussview6/
- 117.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., et al. Gaussian Version 09. [(accessed on 23 January 2023)]. Available online: https://gaussian.com/glossary/g09/
- 118.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pettersen E., Goddard T., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera—a Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 120.Trott O., Olson A.J. Software News and Update AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2009;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Salentin S., Schreiber S., Haupt V.J., Adasme M.F., Schroeder M. PLIP: Fully Automated Protein-Ligand Interaction Profiler. Nucleic Acids Res. 2015;43:W443–W447. doi: 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.