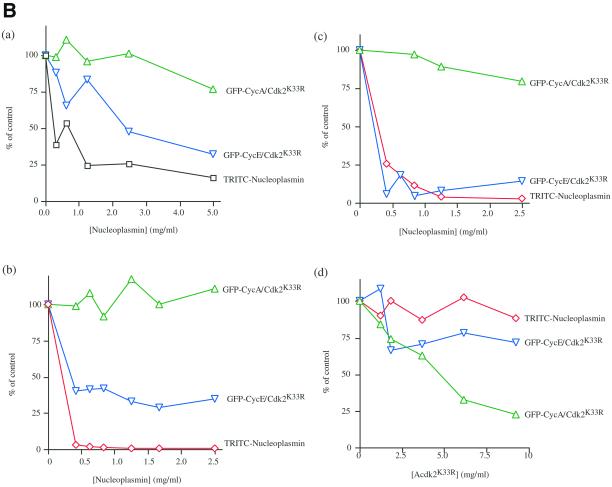
Figure 4.
Nucleoplasmin and cyclin E-GFP/Cdk2K33R but not cyclin A-GFP/Cdk2K33R compete with one another for their nuclear import. Cyclin A/Cdk2K33R is imported into nuclei in vitro. Permeabilized HeLa cells were coincubated with Rch1, importin-β, Ran GDP, an energy-regenerating mixture, and TRITC-labeled nucleoplasmin (a1 and a2) and cyclin A GFP/Cdk2K33R (b1 and b2). Nuclear uptake in the absence (a1 and b1) or presence of 2.5 μM importin-β ΔN44 (a2 and b2) after 30 min of incubation at 22°C was visualized by confocal fluorescence microscopy. (B) Competition experiments among cyclin A, cyclin E, and nucleoplasmin. Permeabilized HeLa cells were coincubated in HeLa cell extract (4.4 mg/ml [a]) or Xenopus egg extract (3.3 mg/ml [b]), with an energy-regenerating mixture, TRITC-labeled nucleoplasmin, and cyclin A-GFP/Cdk2K33R or cyclin E-GFP/Cdk2K33R and increasing concentrations of unlabeled nucleoplasmin. After 30 min of incubation in HeLa extract at 37°C or Xenopus egg extract at 22°C, nuclear uptake of TRITC-labeled nucleoplasmin, cyclin A-GFP/Cdk2K33R, and cyclin E-GFP/Cdk2K33R were visualized by confocal fluorescence microscopy. c and d, permeabilized HeLa cells were incubated under standard conditions using purified proteins in place of cell extracts, with TRITC-labeled nucleoplasmin, cyclin A-GFP/Cdk2K33R, or cyclin E-GFP/Cdk2K33R in the presence of increasing amounts of unlabeled nucleoplasmin (c) or increasing amounts of unlabeled cyclin A/Cdk2(K33R) (d). The average amount of each substrate imported into 100 nuclei was calculated in at least two independent experiments and the mean of these values are shown.